
ij�о���ѧϰС����������ʵ�飺����ˮ�м���������ȩ��Һ���۲쵽��ˮ��ɫ������
[�������]
�������������ԭ����ʲô��
[�������]
����ˮ����ȩ����ȡ����Ӧ��
��___________________________________________________��
��___________________________________________________��
[��Ʒ���]
����һ��������ɫ����Һ������ԡ�
���������ⶨ��Ӧǰ��ˮ��Br2�����ʵ����ͷ�Ӧ��Br�����ӵ����ʵ�����
[ʵ��̽��]
ȡ��0.005mol Br2����Һ10mL������������ȩ��Һʹ����ɫ���ټ������AgNO3��Һ�����ˡ�ϴ�ӡ��������ع�������Ϊ1.88g��
[���������]
�����÷�Ӧǰ��ˮ��Br2�����ʵ���Ϊa mol��
����÷�Ӧ��n(Br��)��0mol����˵����ˮ����ȩ������____________��Ӧ��
����÷�Ӧ��n(Br��)��amol����˵����ˮ����ȩ������____________��Ӧ��
����÷�Ӧ��n(Br��)��2amol����˵����ˮ����ȩ������____________��Ӧ��
����֪CH3COOAg���ܽ���ˮ����ͨ�������ж���ˮ����ȩ������Ӧ������Ϊ___________��
������_________________________________________ _________________��
�䷴Ӧ����ʽΪ_______________________________________________________��
[��˼������]
����һ�Ƿ���У�__________��������_______________________________________��
����ˮ����ȩ�����ӳɷ�Ӧ������ˮ����ȩ����Ϊ���ᡣ
�ӳɷ�Ӧ ȡ����Ӧ ������Ӧ��������Ӧ
����n(Br2):n(Br��)=0.005mol:0.01mol=1:2��������ˮ����ȩ����Ϊ�����ͬʱ��������Br�������ʵ�����Br2�����ʵ�����2��
CH3CHO��Br2��H2O��CH3COOH��2H����2Br��
������ ��ˮ����ȩ����ȡ����Ӧ����ȩ����ˮ��������HBr���ɣ���Һ��������
��������
�����������������ȩ�������в����ͼ���������ˮ��������ȩ�����ӳɷ�Ӧ��
��������ȩ���л�ԭ�ԣ���ˮ���������ԣ�Ҳ���ܽ���ȩ����Ϊ���ᡣ
����÷�Ӧ��n(Br��)��0mol����˵����������ȫ�����ģ���˵����ˮ����ȩ�����˼ӳɷ�Ӧ��
����÷�Ӧ��n(Br��)��amol����˵���������е���ԭ����һ�뱻���ģ���˵����ˮ����ȩ������ȡ����Ӧ��
����÷�Ӧ��n(Br��)��2amol����˵����������ȫ����ԭ���������ӣ���˵����ˮ����ȩ������������Ӧ��
���ɵ��廯�������ʵ�����1.88g��188g/mol��0.01mol�����Ը���n(Br2):n(Br��)=0.005mol:0.01mol=1:2��֪��������ˮ����ȩ����Ϊ�����ͬʱ��������Br�������ʵ�����Br2�����ʵ�����2�������Ը÷�Ӧ��������Ӧ����Ӧ�Ļ�ѧ����ʽ��CH3CHO��Br2��H2O��CH3COOH��2H����2Br����
������ˮ����ȩ����ȡ����Ӧ����ȩ����ˮ��������HBr���ɣ���Һ�������ԣ����Է���һ�Dz����еġ�
���㣺������ˮ����ȩ��Ӧԭ����ʵ��̽��
��������ѧ��һ����ʵ��Ϊ������ѧ�ƣ������л�ѧʵ�鼴��ѧ̽��֮˵�����ݹ۽�����߿�����Ҫ�Կ���̽����ʵ��������Ʊ�ʵ��Ϊ������Щ̽���Ժ��Ʊ���ʵ������⣬�ۺ���ǿ�����ۺ�ʵ������ϵ���ܣ��еĻ��ṩһЩ�µ���Ϣ�����Ҫ��ѧ���������桢ϸ�µ����⣬��ϵ��ѧ����֪ʶ�ͼ��ܣ�����֪ʶ����ȡ�Ǩ�ơ����飬ȫ��ϸ�µ�˼�����ܵó���ȷ�Ľ��ۡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
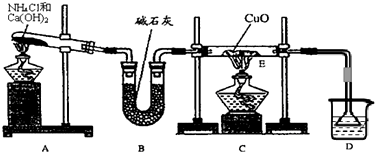
��8�֣�������ˮ����Cl2��H2O��HClO��H+��Cl�������ӣ�Ϊ������ɷ֣�ij�о���ѧϰС����������ʵ�飬���������ʵ�飬��Ҫ����ա�
��1��ȡ����������ˮ���Թ��У�{007}����̼��Ʒ�ĩ�����������ݲ�������˵��������
�ijɷ���HCl��HCl���ֳ� �ԡ�
��2��ȡ����������ˮ���Թ��У�����AgNO3��Һ�������а�ɫ�����������������õ��� ��
��3��ȡ����������ˮ���Թ��У�����һ���ֽ�����ֺܿ���ɫ���������õ��� ��
��4��ȡ����������ˮ���Թ��У�����FeCl2��Һ�����ֺܿ��ƣ������õijɷ���Cl2��˵���������� �ԡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������������غ���¡��һ����ѧ�߶�6���¿���ѧ������������ ���ͣ�ʵ����
ij�о���ѧϰС����������ʵ�飺����ˮ�м���������ȩ��Һ���۲쵽��ˮ��ɫ������
��1��[�������]
�������������ԭ����ʲô��
��2��[�������]
����ˮ����ȩ����ȡ����Ӧ��
��___________________________________________________��
��___________________________________________________��
��3��[��Ʒ���]
����һ��������ɫ����Һ������ԡ�
���������ⶨ��Ӧǰ��ˮ��Br2�����ʵ����ͷ�Ӧ��Br�����ӵ����ʵ�����
��4��[ʵ��̽��]
ȡ��0.005mol Br2����Һ10mL������������ȩ��Һʹ����ɫ���ټ������AgNO3��Һ�����ˡ�ϴ�ӡ��������ع�������Ϊ1.88g��
��5��[���������]
�����÷�Ӧǰ��ˮ��Br2�����ʵ���Ϊa mol��
����÷�Ӧ��n(Br��)��0 mol����˵����ˮ����ȩ������____________��Ӧ��
����÷�Ӧ��n(Br��)��a mol����˵����ˮ����ȩ������____________��Ӧ��
����÷�Ӧ��n(Br��)��2a mol����˵����ˮ����ȩ������____________��Ӧ��
����֪CH3COOAg���ܽ���ˮ����ͨ�������ж���ˮ����ȩ������Ӧ������Ϊ___________��������________________________________________��
�䷴Ӧ�����ӷ���ʽΪ__________________________________________________��
��6��[��˼������]
����һ�Ƿ���У�__________��������_______________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com