
 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| Ԫ�� | Na | Mg | Al | Si | P | S | Cl |
| �縺�� | 0.9 | 1.2 | 1.5 | 1.8 | 2.1 | 2.5 | 3.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����AgCl����Һ�м���������NaI��Һ������������ |
| B��CCI4��NH3�������и�ԭ������������8���ӽṹ |
| C����������Ԫ��R2+��M+�ĵ��Ӳ�ṹ��ͬ���������R>M |
D����״���£�N2��O2�Ļ����11.2L����ԭ������Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��Na+��Fe3+��Cl ��SCN ��SCN �ܴ���������ͬһ��Һ�� �ܴ���������ͬһ��Һ�� |
B��̼��������Һ������������������Һ��Ϻ������ӷ���ʽΪ��HCO3 + Ba2+ + OH + Ba2+ + OH ="=" BaCO3��+H2O ="=" BaCO3��+H2O |
C����R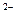 ��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M ��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M |
D����Xԭ���е�������Ϊa��������Ϊb����X��������Ϊ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
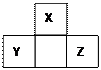
| A��X��Y��Z���߾�Ϊ����Ԫ�� |
| B��X��Y��Z���ߺ��������֮��Ϊ40 |
| C��X��Y��Y��Z�����γ����ӻ����� |
| D��Y��Zֻ���γ�һ�ֹ��ۻ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| | ���ж� | ���ж� |
| A | ij����������ˮ�ܵ��� | �û������ǵ���� |
| B | ij���̷��������仯 | �ù��̷�����ѧ��Ӧ |
| C | ��ԭ�Ӻ�������������� | �ƵĽ����Ա���ǿ |
| D | ����Ԫ��ͬһ���� | ����Ԫ�ص�ԭ��������������ͬ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���٢� | B��ֻ�Т� | C���ڢ� | D���ۢ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com