
����Ŀ�������������ڹ�ũҵ�����ж�����ҪӦ�á�
��1�������£�N2H4������������ĵ��⻯�
��֪��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1��N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ_________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=____����K1��K2��ʾ����
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2������NH3��O2��ȡN2H4���Ȼ�ѧ����ʽΪ_________���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=____����K1��K2��ʾ����
��2������2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��������˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����____������ĸ���ţ���
A��c(CO)=c(CO2) B�������л��������ܶȲ���
C��v(N2)��=2v(NO)�� D�������л�������ƽ��Ħ����������
��ͼ1Ϊ�����ڵ�ѹǿ��P������ʼѹǿ��P0���ı�ֵ��P/P0����ʱ�䣨t���ı仯���ߡ�0��5min�ڣ��÷�Ӧ��ƽ����Ӧ����v(N2)= ____��ƽ��ʱNO��ת����Ϊ____��
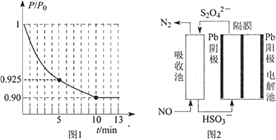
��3��ʹ�ü�ӵ绯ѧ���ɴ���ȼú�����е�NO��װ����ͼ2��ʾ����֪���ص�����������Һ��pH��4��7֮�䣬д�������ĵ缫��Ӧʽ_________�������ӷ���ʽ��ʾ���ճ��г�ȥNO��ԭ��__________________��
���𰸡� 4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol ![]() D 0.006 mol��L��1��min��1 80% 2HSO3����2e����2H��=S2O42����2H2O 2NO��2S2O42����2H2O=N2��4HSO3��
D 0.006 mol��L��1��min��1 80% 2HSO3����2e����2H��=S2O42����2H2O 2NO��2S2O42����2H2O=N2��4HSO3��
��������(1)��4NH3(g)��3O2(g) ![]() 2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g)
2N2(g)��6H2O(g) ��H1=��541.8kJ/mol����ѧƽ�ⳣ��ΪK1����N2H4(g)��O2(g) ![]() N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=
N2(g)��2H2O(g) ��H2=��534kJ/mol����ѧƽ�ⳣ��ΪK2�����ݸ�˹���ɣ�����-����2�ã�4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=(��541.8kJ/mol)-(��534kJ/mol)��2=��526.2kJ/mol���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=![]() ���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol��
���ʴ�Ϊ��4NH3(g)��O2(g) = 2N2H4(g)��2H2O(g) ��H=��526.2kJ/mol�� ![]() ��
��
(2)����2NO(g)��2CO(g) ![]() N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
N2(g)��2CO2(g)����һ���¶��£���1L�ĺ����ܱ������г���0.1molNO��0.3molCO����Ӧ��ʼ���С�
��A��c(CO)=c(CO2)������ʾŨ�ȱ仯�������ж��Ƿ�Ϊƽ��״̬����A����B����Ӧ��������������䣬������䣬�����л��������ܶ�ʼ�ղ��䣬�����ж��Ƿ�Ϊƽ��״̬����B����C��v(N2)��=2v(NO)����ʾ��Ӧ����2v(N2)��=v(NO)�����ű�ʾ���淴Ӧ������ȣ���C����D���÷�Ӧ������������ʵ��������仯�ķ�Ӧ�������л�������ƽ��Ħ����������ʱ��ʾ��������ʵ������䣬 ˵����ƽ��״̬����D��ȷ����ѡD��
�����������ڵ�ѹǿ(P)����ʼѹǿ(P0)�ı�ֵ(P/P0)��ʱ��(t)�ı仯���ߣ�0��5min�ڣ� ![]() =0.925�����ݰ���٤�����ɼ������ۣ�
=0.925�����ݰ���٤�����ɼ������ۣ� ![]() =0.925��ƽ��ʱ
=0.925��ƽ��ʱ![]() =0.90��
=0.90��
2NO(g)�� 2CO(g) ![]() N2(g)��2CO2(g)
N2(g)��2CO2(g)
��ʼ(mol) 0.1 0.3 0 0
��Ӧ 2x 2x x 2x
5min��ƽ�� 0.1-2x 0.3-2x x 2x
5min![]() =0.925�����x=0.03mol��v(N2)=
=0.925�����x=0.03mol��v(N2)=  = 0.006mol��L��1��min��1��ƽ��ʱ��
= 0.006mol��L��1��min��1��ƽ��ʱ�� ![]() =0.90�����x=0.04mol��NO��ת����=
=0.90�����x=0.04mol��NO��ת����=![]() ��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
��100%=80%���ʴ�Ϊ��0.006 mol��L��1��min��1��80%��
(3)����������ԭ��Ӧ����������������ӣ��õ��ӣ����������������ӣ��缫��ӦʽΪ��2HSO3-+2e-+2H+�TS2O42-+2H2O����������������һ����������������ԭ��Ӧ�����ɵ��������ӷ�Ӧ����ʽΪ��2NO+2S2O42-+2H2O�TN2+4HSO3-���ʴ�Ϊ��2HSO3-+2H++2e-=S2O42-+2H2O��2NO+2S2O42-+2H2O=N2+4HSO3-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���������߷ֱ��ʾ1g C3H6���塢1g M��������ͬ�����������ѹǿ���¶ȵĹ�ϵ���Ը���ͼ�ж�M���������

A��PH3 B��N2 C��C3H4 D��N2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ճ������е����������뻯ѧ��Ӧ�йأ�����������������ԭ��Ӧ�ص��� (����)
A. ͭ�������ϳ���ͭ��[Cu2(OH)2CO3] B. ��������������������ը
C. ����ʯ�������긯ʴ�ٻ� D. ���ʲ˵�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����Һ�еĸ������ʵ����ʵ���Ũ�Ⱦ�Ϊ0.1 mol/L����H2S��Һ����KHS��Һ����K2S��Һ����H2S��KHS�����Һ�� ����˵����ȷ����
A. ��ҺpH�Ӵ�С��˳���ǣ��� > �� > �� > ��
B. ��KHS��Һ���У�c(H+) + c(K+) = c(OH-) + c(HS-) + c(S2-)
C. c(H2S)�Ӵ�С��˳���ǣ��� > �� > �� > ��
D. ��H2S��KHS�����Һ���У�c(H2S) + c(HS-) + c(S2-) = 2c(K+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�缫��ÿͨ��96500 C�ĵ����ͻ���1 mol���ӷ���ת�ơ���ȷ�������������ڶ��Ե缫���ԶƲ���ʽ�����Ľ�������������ȷ����������ͨ�����صĵ�����ʵ�ʲ����У������������ƣ���ͼ��ʾ������˵������ȷ����

A. ��Ҫ�ⶨ��ⱥ��ʳ��ˮʱͨ���ĵ������ɽ������������е������������ص��������������������Դ�ĸ���������
B. �������ǰ�������������仯���ý������ij�����Ϊ108.0 mg�����������ͨ�����صĵ���Ϊ96.5 C
C. ʵ���У�Ϊ�˱������ܽ�����п��ܲ����Ľ����������������������²������������缫��������һ���ռ���������û���ռ����������������ƫ�ߡ�
D. �������е�����Ӧ���Դ�����������������������ĵ缫��Ӧ�ǣ�Ag+ + e- = Ag
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ࡢ��֬����������������Ҫ��Ӫ�����ʡ������й����������ʵ�˵����ȷ����
A.������Ȼ�߷��ӻ�����B.��ֻ��C��H��O����Ԫ�����
C.����������ֵ��ߵ�Ӫ������D.���ۡ���֬����������һ�������¾��ܷ���ˮ�ⷴӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݷ�ӦCu+2H2SO4��Ũ��![]() CuSO4+SO2��+2H2O���ش��������⣺
CuSO4+SO2��+2H2O���ش��������⣺
(1)����˫���ŷ����������ת�Ƶķ������Ŀ________________��
(2)���û�ѧʽд���÷�Ӧ�еĻ�ԭ����__________����������__________������������__________��
(3)��ԭ���ͻ�ԭ�������ʵ���֮��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
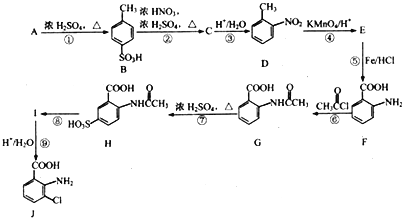
����Ŀ���Է�����A(C7H8)Ϊ��Ҫԭ�Ϻϳ���Ҫ��ҽҩ�м���J������ͼ���£�
��֪����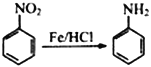 (-NH2���ڱ�ǿ����������)
(-NH2���ڱ�ǿ����������)
��![]()
��![]()
�ش��������⣺
(1)A�Ľṹ��ʽΪ__________��������в�ͬ��ѧ��������ԭ�ӵĸ�����Ϊ__________��
(2)B��C �Ļ�ѧ����ʽΪ_____________________����Ӧ�ܵķ�Ӧ����Ϊ__________��
(3)��Ӧ�������Լ�������Ϊ___________��E�к��������ŵ�����Ϊ_____________��
(4)��Ӧ�͢ߵ�˳���Ƿ���Խ�������?__________(ѡ��ǡ���)�������ޡ��Ჽ�ķ�Ӧ�����з�Ӧ����ҪĿ����_____________________��
(5)K��J��ͬ���칹�壬�䱽���ϵ�ȡ������J����ͬ��λ�ò�ͬ����K���ܵĽṹ��__________�֡�
(6)�����������ʾ�ϳ�·�ߣ��Ա���![]() Ϊԭ��(���Լ���ѡ)������Ʊ�
Ϊԭ��(���Լ���ѡ)������Ʊ�![]() �ĺϳ�·��__________���ϳ�·��ʾ�����£�CH3CH2OH
�ĺϳ�·��__________���ϳ�·��ʾ�����£�CH3CH2OH![]() CH2=CH2
CH2=CH2![]()
![]()
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com