
����Ŀ��Ϊ�˺������û�ѧ�ܣ�ȷ����ȫ���������������Ҫ��ֿ��ǻ�ѧ��Ӧ���ʱ䣬����ȡ��Ӧ��ʩ����ѧ��Ӧ���ʱ�ͨ����ʵ����вⶨ��Ҳ�ɽ����������㡣
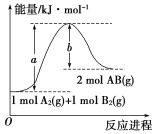
(1)��֪��ѧ��ӦA2(g)��B2(g)===2AB(g)�������仯��ͼ��ʾ��д���÷�Ӧ���Ȼ�ѧ����ʽ____��
(2)ʵ���ã�1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ����������д���Ҵ�ȼ�յ��Ȼ�ѧ����ʽ��_______��
(3)���ݸ�˹���ɿ��Զ�ijЩ����ͨ��ʵ��ֱ�Ӳⶨ�Ļ�ѧ��Ӧ���ʱ�����������㡣�����������Ȼ�ѧ����ʽ�����㷴Ӧ2C(s)��2H2(g)��O2(g)===CH3COOH(l)���ʱ�Ϊ_________��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)===CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)===H2O(l) ��H3����285.8 kJ��mol��1
O2(g)===H2O(l) ��H3����285.8 kJ��mol��1
���𰸡�A2(g)��B2(g)===2AB(g)����H��(a��b) kJ��mol��1 C2H5OH(l)��3O2(g)===2CO2(g)��3H2O(l)����H����1366.2 kJ��mol��1 ��488.3 kJ��mol��1
��������
��1��������H=��Ӧ��Ļ��-�����������㣻
��2��1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ�����������1mol�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7��46 kJ=1366.2 kJ��������д���Ȼ�ѧ����ʽ��
��3����CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
��ϸ�˹���ɿ�֪���ڡ�2+�ۡ�2-���õ�2C��s��+2H2��g��+O2��g���TCH3COOH��l�����Դ������
��1����ͼ������H=��Ӧ����-��������=��a-b��kJ��mol��1 ����Ӧ���Ȼ�ѧ����ʽ��A2(g)��B2(g)=2AB(g)����H��(a��b) kJ��mol��1��
��2��1 g�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7 kJ�����������1mol�Ҵ��������г��ȼ�����ɶ�����̼��Һ̬ˮ�ͷų�29.7��46 kJ=1366.2 kJ��������д���Ȼ�ѧ����ʽ��C2H5OH(l)��3O2(g)=2CO2(g)��3H2O(l)����H����1366.2 kJ��mol��1 ��
��3����CH3COOH(l)��2O2(g)=2CO2(g)��2H2O(l)����H1����870.3 kJ��mol��1
��C(s)��O2(g)=CO2(g)����H2����393.5 kJ��mol��1
��H2(g)��![]() O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
O2(g)=H2O(l) ��H3����285.8 kJ��mol��1
��ϸ�˹���ɿ�֪���ڡ�2+�ۡ�2-���õ�2C��s��+2H2��g��+O2��g���TCH3COOH��l��������H=��-393.5kJ��mol��1����2+��-285.8kJ��mol��1����2-��-870.3kJ��mol��1��=-488.3kJ��mol��1��


 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ�У���ȷ����
A�������ȼ����Ϊ890��3kJ/mol�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ��
CH4��g��+2O2��g��==CO2��g��+2H2O��g����H=-890��3kJ/mol
B��500����30MPa�£���0��5mol N2��1��5molH2�����ܱյ������г�ַ�Ӧ����NH3��g��������19��3kJ�����Ȼ�ѧ����ʽΪ��N2��g��+3H2��g��![]() 2NH3��g����H=-38��6kJ/mol
2NH3��g����H=-38��6kJ/mol
C��NaOH��s��+1/2 H2SO4��Ũ��==1/2Na2SO4��aq��+H2O��l����H=-57��3kJ/mol
D��2��00gC2H2������ȫȼ������Һ̬ˮ�Ͷ�����̼���壬�ų�99��6kJ���������÷�Ӧ���Ȼ�ѧ����ʽΪ��2C2H2��g��+5O2��g��==4CO2��g��+2H2O��l����H=-2589��6kJ/moL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����500mL NaOH��Һ��Ͷ��10.8g Al������ǡ����ȫ��Ӧ�����㣺
(1)Al�����ʵ���__________��
(2)�μӷ�Ӧ��NaOH�����ʵ���__________��NaOH��Һ�����ʵ���Ũ��___________��
(3)���ɵ�H2�ڱ�״���µ����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�ý���ʵ�飬��ʼʱ��a��b����Һ����ƽ���ܷ�ã�����һ��ʱ�䡣����˵������ȷ����( )

A. a�ܷ���������ʴ��b�ܷ������ⸯʴ
B. һ��ʱ���a��Һ�����b��Һ��
C. a����Һ��pH����b����Һ��pH��С
D. a��b����������ͬ�ĵ缫��Ӧʽ��Fe��2e��===Fe2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ж��֣��������ȿ���������ˮ������84����Һ���ڼ�ͥ�����ݵ�������
I.ʵ���ҿ����������ƹ�����Ӧ�Ʊ�ClO2��2NaClO2+Cl2=2ClO2+2NaCl��װ����ͼ��ʾ��
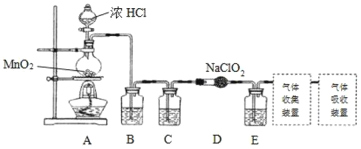
��1��װ��A�У�ʢװŨ�������������Ϊ___����ʼ��Ӧ��Բ����ƿ�ڷ����ķ�Ӧ�����ӷ�Ӧ����ʽΪ��__��
��2����֪���³�ѹ�£�ClO2��Cl2�����壬�ڲ�ͬ�ܼ����ܽ��������ʾ��B��C��Eװ���е��Լ�������___�����ţ�
ClO2 | Cl2 | |
ˮ | ��������ˮ | �� |
CCl4 | ���� | ���� |
a.Ũ���� b.����ʳ��ˮ c.NaOH��Һ d.CCl4
��3��ʹ��ClO2�ڸ�����ˮ�����Ĺ����л�����к��ĸ��������������ClO2-��������Fe2+����ȥ������֪ClO2-��Fe2+��pH=5��7���������ܿ��ٷ�Ӧ�������γɺ��ɫ��������ClO2-��ԭ��Cl-��Fe2+����ClO2-�����ӷ���ʽΪ__��
II.ijͬѧ�ڼ������Ƴ�����84����Һ���ɷ֣�NaClO��ˮ����ͬ������Һ����Ҫ������6V��ѹ������֧ľ��Ǧ�ʡ��ϴ���ˮƿ��ʳ�Ρ�����ֽ����Ե������С���ȡ�
��4��ʵ����̣���һ�������Ĵ���ˮƿ�й�����ƿ����ˮ��������3��ζ��ʳ�Σ�������ֽ����Ǧ�����ɵĵ缫���ã������봿��ˮƿ�У�ʹ�缫ǡ�ÿ���ƿ�ڣ�װ����ͼ����ͨ��Դ���Կ���һ��缫������������һ��缫��ϸС�����ݲ�������д���õ缫��ӦʽΪ��___������ͨ��Լ3Сʱ����ԭ������������ĵ缫����Ҳ��ʼ����һ������ϸС���ݣ��˵缫��ʱ�ĵ缫��Ӧʽ___�������������ֹͣͨ�硣

��5���ø÷����Ʊ�����Һ���ܻ�ѧ����ʽ�ǣ�___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����º��������£����������в��ǿ��淴Ӧ2A��g��+3B��s��![]() 2C��g���ﵽƽ��ı�־���ǣ� ��
2C��g���ﵽƽ��ı�־���ǣ� ��
��C������������C�ķֽ�������ȣ��ڵ�λʱ������amol A��ͬʱ����1.5amol B���۸���ֵ�Ũ�Ȳ��ٱ仯���ܻ��������ܶȲ��ٱ仯���ݻ���������ѹǿ���ٱ仯�������������ʵ������ٱ仯����������ƽ��Ħ���������ٱ仯����A��B��C�ķ�����Ŀ��Ϊ2��3��2��
A. �ݢޢ�B. �ڢݢ�C. �٢ۢ�D. �ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ȼ�ѧ����ʽ��������ȷ����
A. 1 molҺ̬����������������ȫȼ������ˮ�������ų�642 kJ��������N2H4(l)+O2(g) ===N2(g)+2H2O(g) ��H��+642 kJ��mol��1
B. 12 gʯīת��ΪCOʱ���ų�110.5 kJ��������2C(ʯī��s)+O2(g) ===2CO(g) ��H����110.5 kJ��mol��1
C. ��֪��H2(g)+ ![]() O2(g) ===H2O(l) ��H����286 kJ��mol��1������2H2O(l) ===2H2(g)+O2(g)����H��+572 kJ��mol��1
O2(g) ===H2O(l) ��H����286 kJ��mol��1������2H2O(l) ===2H2(g)+O2(g)����H��+572 kJ��mol��1
D. ��֪N2(g)+3H2(g) ![]() 2NH3(g) ��H����92.4 kJ��mol��1������һ�����������ܱ������г���0.5 mol N2(g)��1.5 mol H2(g)��ַ�Ӧ�ų�46.2 kJ������
2NH3(g) ��H����92.4 kJ��mol��1������һ�����������ܱ������г���0.5 mol N2(g)��1.5 mol H2(g)��ַ�Ӧ�ų�46.2 kJ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ȩ�������Ȼ����������� ѡ���ڻ�� ��ZrTe5��Ϊ������֤����ά���ӻ���ЧӦ�� �������˽�������Ե���ת����Te���ڢ�AԪ�ء��ش��������⣺
(1)�(Zr)�ļ����Ų�ʽΪ[Kr]4d25s2���ԭ����d����ϵĵ�������___��Zr2+�ļ۵����Ų�ͼ��___��
(2)O��Se��Te�ĵ�һ�������ɴ�С��˳����___��H2O��H2Se��H2Te�ķе��ɸߵ��͵�˳����___��
(3)H2Te��CO2 ��Ϊ��ԭ�ӷ��ӣ������ǵļ��Dz��ϴ������ӻ�������۽��ͣ�������___��
(4) [Zr(C2H5O)2]2+��Zr4+�γɵ�һ�������ӣ����е���λԭ����___(�����)�� 1��[Zr(C2H5O)2]2+�����к����ۼ�����Ŀ��___��
(5)�����������һ���˹��ϳɵ��������Ӳ�ȼ��ߣ��������մɺ��ͻ���ϣ��侧���ṹ��ͼ��ʾ��Zrԭ�ӵ���λ����___���������о��������������ԭ�Ӽ�ľ���Ϊanm������������ﯵ��ܶ�Ϊ___ g/cm3��
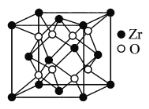
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ��ȷ����
A.Al(OH)3����������NaOH��Һ��Al(OH)3��OH����AlO2����2H2O
B.Na2O2��H2O��Ӧ��Na2O2��H2O��2Na����2OH����O2��
C.������ˮ�ķ�Ӧ��Cl2��H2O��2H����Cl����ClO��
D.�����еμӰ�ˮ��H����OH����H2O
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com