
| ���� | �� |

| 1000��w |
| M |
| 1000��0.91��25% |
| 17 |
| amol |
| 4 |
| һ������ |
| 4L��5 |
| 4��0.20 |
| 4L |
| 4L+25L |
| 5 |
| 4 |
| 1.5mol |
| 20.0mol |
| 18.0+1.5 |
| 20 |
| ���� |
| �� |
| ��+�� |
| 2 |
| 5 |
| 4 |
 ��
�� ��
��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ���������������ѧ����ĩ��ѧ�������л�ѧ�Ծ��������棩 ���ͣ�������
��1�������ڹ�ũҵ���Ź㷺����;����֪25%��ˮ���ܶ�Ϊ0.91 g/cm3��5%��ˮ���ܶ�Ϊ0.98 g/cm3��
������100mL 2.5mol/L��ˮ��ҪŨ��Ϊ25%��ˮ______mL������2λС������
��������������Һ�������ϣ����ð�ˮ��Һ������������_____________��
A������15% B������15% C����15% D��������
��֪��4NH3+O2 4NO+6 H2O��4NO+3O2+2H2O
4NO+6 H2O��4NO+3O2+2H2O 4HNO3
4HNO3
��2����������������������Ϊ0.20���������������Ϊ0.80��
��a mol NO��ȫת��ΪHNO3��������Ҫ����_____________mol��
��ΪʹNH3ǡ����ȫ����ΪNO������������������а��������������С����ʾ��Ϊ_____________
������2λС������
��3��20.0 mol NH3�ÿ����������������������Ϊ��NO 18.0 mol��O2 12.0 mol��N2 150.0 mol��һ�������ᣬ�Լ������ɷ֣�������NO��O2�����ϣ������㰱ת��ΪNO��HNO3��ת���ʡ�
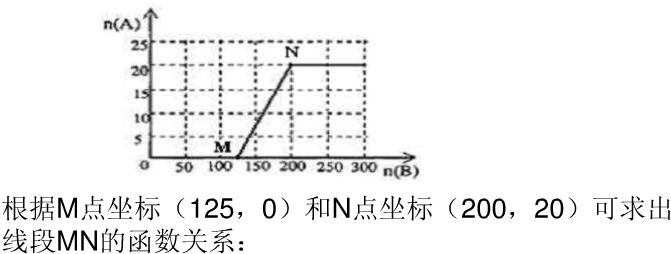
��4��20.0 mol NH3��һ����������ַ�Ӧ����ת��Ϊ���ᡣͨ�����㣬��ͼ�л���HNO3�����ʵ���n(A)�Ϳ��������ʵ���n (B)��ϵ���������ߡ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ����������߿�һģ��ѧ�Ծ��������棩 ���ͣ������
�����ڹ�ũҵ���Ź㷺����;����֪25%��ˮ���ܶ�Ϊ0.91 g/cm3��5%��ˮ���ܶ�Ϊ0.98 g/cm3��
1����1������100mL 2.5mol/L��ˮ��ҪŨ��Ϊ25%��ˮ______mL������2λС������
��2��������������Һ�������ϣ����ð�ˮ��Һ������������_____________��
A������15% B������15% C����15% D��������
2����֪��4NH3+O2 4NO+6NO��4NO+3O2+2H2O
4NO+6NO��4NO+3O2+2H2O 4HNO3
4HNO3
��������������������Ϊ0.20���������������Ϊ0.80��
��1��a mol NO��ȫת��ΪHNO3��������Ҫ����_____________mol��
��2��ΪʹNH3ǡ����ȫ����ΪNO����-������������а��������������С����ʾ��Ϊ_____________ ������2λС������
��3��20.0 mol NH3�ÿ����������������������Ϊ��NO 18.0 mol��O2 12.0 mol��N2 150.0 mol��һ�������ᣬ�Լ������ɷ֣�������NO��O2�����ϣ������㰱ת��ΪNO��HNO3��ת���ʡ�
��4��20.0 mol NH3��һ����������ַ�Ӧ����ת��Ϊ���ᡣͨ�����㣬��ͼ�л���HNO3�����ʵ���n(A)�Ϳ��������ʵ���n (B)��ϵ���������ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 4NO+6H2O��4NO+3O2+2H2O��4HNO3
4NO+6H2O��4NO+3O2+2H2O��4HNO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ����������������ϣ���ĩ�߸߿���ѧһģ�Ծ��������棩 ���ͣ������
 4NO+6H2O��4NO+3O2+2H2O��4HNO3
4NO+6H2O��4NO+3O2+2H2O��4HNO3
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com