
(14��)��������׳�PP�ۣ�Ϊǿ�������������л���ͷų�����������������ɱ��ϸ�������ã���ɱ��������ǿ��ijѧ������ʵ��������1 L 0.06 mol/L KMnO4ϡ��Һ��������ϴ�˿ڡ�
(1)ʵ�����������������ƽ��ȡKMnO4���������Ϊ__________g��
(2)�������������ͼ��ʾ������ͼ��ʾ����Ӧ����ͼ��__________(��ѡ����ĸ)֮�䡣
A������ۡ���B������ڡ���C�������
(3) ���ƹ�������Ҫ�õ���������:������ƽ��ҩ�ס��ձ��� �� ��
(4)����ͬѧ��������Һʱ�����������²���������ʹ������ҺŨ��ƫ�͵IJ���
��_______________(��ѡ����ĸ)��
A������KMnO4����ʱ��ָ��ƫ���ұ�
B����KMnO4��������ձ����ܽ⣬��ȴ��ת����������������ˮ������ƿ��
C������ʱ�����ӿ̶���
D����ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ٵμ���������ˮ���̶��ߴ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��������ˮһ�и�����ѧ����ĩģ�⻯ѧ�Ծ����������� ���ͣ������
(14��)
��1�������±��и�����Ų����ɣ����˹����Ų���22��ӦΪ ��
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| C2H4 | C2H6 | C2H6O | C2H4O2 | C3H6 | C3H8 | C3H8O | C3H6O2 | C4H8 | C4H10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ��̨�ڶ���ѧ����11��ģ���⻯ѧ�Ծ� ���ͣ�ʵ����
(14��)��������׳�PP�ۣ�Ϊǿ�������������л���ͷų�����������������ɱ��ϸ�������ã���ɱ��������ǿ��ijѧ������ʵ��������1 L 0.06 mol/L KMnO4ϡ��Һ��������ϴ�˿ڡ�
(1)ʵ�����������������ƽ��ȡKMnO4���������Ϊ__________g��
(2)�������������ͼ��ʾ������ͼ��ʾ����Ӧ����ͼ��__________(��ѡ����ĸ)֮�䡣
A������ۡ���B������ڡ���C�������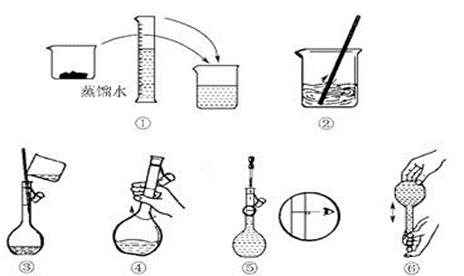
(3) ���ƹ�������Ҫ�õ���������:������ƽ��ҩ�ס��ձ��� �� ��
(4)����ͬѧ��������Һʱ�����������²���������ʹ������ҺŨ��ƫ�͵IJ���
��_______________(��ѡ����ĸ)��
A������KMnO4����ʱ��ָ��ƫ���ұ�
B����KMnO4��������ձ����ܽ⣬��ȴ��ת����������������ˮ������ƿ��
C������ʱ�����ӿ̶���
D����ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ٵμ���������ˮ���̶��ߴ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ������ѧ����4��˫����ϰ��ѧ�Ծ����������� ���ͣ������
(14��) �����������������Լ�����Ӱ���ȣ����Ʊ��������£�
������(NH4)2Fe(SO4)2 6H2O��Һʱ���������ϡ���ᣬĿ���� ��
6H2O��Һʱ���������ϡ���ᣬĿ���� ��
�ƽ��ƵõIJ�Ʒ����������н������ط������������ͼ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�������
����C��ʱ������Ļ�ѧʽΪ ��
�����о�ѧ����ʵ�������������ɫ�����H2�����ղ�����Ҳ�����Ĵ����������ɣ����������һ������ʽ����������ʵ�� ��
����ȡ�������146����ˮ���FeC2O41.44g����ij��յ��ܱ������У��ٳ���0.04molCO��������1100�棬����FeO(s)+CO(g) Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
Fe(s)+CO2(g)��Ӧƽ�ⳣ��K=0.4����÷�Ӧ��ƽ��ʱ��FeO��ת����Ϊ���٣� ��
��3������þ�ڹ�������������Ҫ���ã�����MgCl2Ϊԭ�ϻ�ȡ���¶Ⱥ�ѹǿP(HCl)g��MgCl2��6H2O�����ȷֽ�����Ӱ����ͼ��ʾ�������ͼ��ش��������⣺
��д��P(HCl)g = 0.25MPa���¶ȴ�300�����ߵ�550��ʱ��Ӧ�Ļ�ѧ����ʽ ��
��ʵ�������У���MgCl2��6H2O������ȵ�600��Ĺ����м����ò�����ˮMgCl2����ԭ���� ����Ҫ�õ���ˮMgCl2���ȡ�Ĵ�ʩ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��ʡ����11��ģ���⻯ѧ�Ծ� ���ͣ�ʵ����
(14��)��������׳�PP�ۣ�Ϊǿ�������������л���ͷų�����������������ɱ��ϸ�������ã���ɱ��������ǿ��ijѧ������ʵ��������1 L 0.06 mol/L KMnO4ϡ��Һ��������ϴ�˿ڡ�
(1)ʵ�����������������ƽ��ȡKMnO4���������Ϊ__________g��
(2)�������������ͼ��ʾ������ͼ��ʾ����Ӧ����ͼ��__________(��ѡ����ĸ)֮�䡣
A������ۡ���B������ڡ���C�������
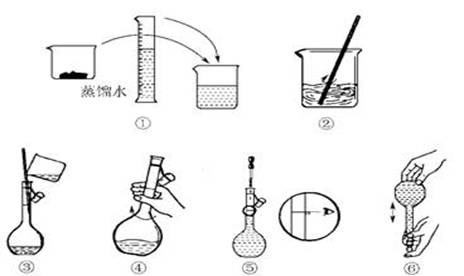
(3) ���ƹ�������Ҫ�õ���������:������ƽ��ҩ�ס��ձ��� �� ��
(4)����ͬѧ��������Һʱ�����������²���������ʹ������ҺŨ��ƫ�͵IJ���
��_______________(��ѡ����ĸ)��
A������KMnO4����ʱ��ָ��ƫ���ұ�
B����KMnO4��������ձ����ܽ⣬��ȴ��ת����������������ˮ������ƿ��
C������ʱ�����ӿ̶���
D����ҡ�Ⱥ�����ҺҺ����ڿ̶��ߣ��ٵμ���������ˮ���̶��ߴ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com