
���� ��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���Բ���˳��������������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��������Ҫ������������ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ���ݴ�ѡ��
��2�������������������ƹ����������Ȼ������ձ���������ѡ������������������������
��3������ƿ��ʹ��ǰ�����©��
��4������c=$\frac{n}{V}$��������ʵ����ʵ���n����Һ�����V�ı仯��������������
��� �⣺��1���������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪��ȷ�IJ���˳���ǣ��ڢ٢ۢ�ݢޢߢܣ��������Ʋ����Ǽ��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��֪���������Ϊ����ƽ��ҩ�ס����������ձ�������ƿ�ͽ�ͷ�ιܣ�����ƿֻ��һ���̶��ߣ���ֻ�����ƺ��������Ӧ���������Һ������ʵ������480mL����ƿ����Ӧѡ��500mL������ƿ���ʴ�Ϊ���ۢ�ݢޢߢܣ���ͷ�ιܣ�500mL��
��2������ʵ������450mL����ƿ����Ӧѡ��500mL������ƿ�������Ƴ�500mL��Һ����������������Ƶ�����m=CVM=1mol/L��0.5L��40g/mol=20.0g��
�����ձ�������Ϊ25.6g������������������Ϊ45.6g����������С����������Ϊ1.0g���ʿ���ѡ�õ����������Ϊ45g��Ȼ����������0.6g�����ʴ�Ϊ��45g��0.6g��
��3������ƿ��ʢװҺ�������������ʹ�õĹ�����Ҫ��תҡ�ȣ�����ʹ��ǰ�����©���ʴ�Ϊ����©��
��4����û��ϴ���ձ��Ͳ��������ᵼ�����ʵ���ʧ����Ũ��ƫ�ͣ��ʢٴ���
��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�죬�ʢڴ���
�۶���ʱ���ӿ̶��ߣ��ᵼ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ���ȷ��
��δ��ȴ�����¾ͽ���Һת�Ƶ�����ƿ�����ݣ�����ȴ����Һ���ƫС����Ũ��ƫ�ߣ��ʢ���ȷ��
�ݶ��ݺ�����ƿ������ҡ�ȣ����ú�Һ����ڿ̶����������ģ��ټ�ˮ���̶��ᵼ��Ũ��ƫ�ͣ��ʢݴ���
��ѡ�ۢܣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƹ����еļ���������������ڻ�������Ŀ���ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ۢ� | B�� | �٢� | C�� | �ڢ� | D�� | �٢ۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��ͼ�Ǽ���ȼ�ϵ��ԭ��ʾ��ͼ���ش��������⣺
��ͼ�Ǽ���ȼ�ϵ��ԭ��ʾ��ͼ���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
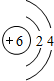
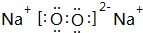 �����ڵĻ�ѧ�����������Ӽ������ۼ��������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2=2Na2CO3+O2��
�����ڵĻ�ѧ�����������Ӽ������ۼ��������Ӧ�Ļ�ѧ����ʽ��2Na2O2+2CO2=2Na2CO3+O2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ˮ--��ɫƿ�������� | B�� | �����--��Ƥ��������ƿ | ||
| C�� | ����������--���ƿ��ú���� | D�� | ϡ����--ϸ��ƿ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ʹ�õ���Ҫ�����������ƾ��ơ�������������Ȧ������̨ | |
| B�� | ��Һʱ����Һ©���е��²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� | |
| C�� | ����ʱ��������ĩ��Ӧ������������ֽ�� | |
| D�� | �ù��Ϊ10 mL����Ͳ��ȡ6.20 mL��Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��������NaOHˮ��Һ��ϼ��� | |
| B�� | �״���Ũ�����ϼ��� | |
| C�� | �Ҵ���Ũ�����ϼ��� | |
| D�� | 2-�嶡����NaOH���Ҵ���Һ��ϼ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ܷ����ֽⷴӦ | B�� | ������ˮ��ɸ����� | ||
| C�� | ������ֻ��һ��ȩ�� | D�� | �����Ƿ����к�̼ԭ�������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com