
��13�֣���1��ָ������ʵ����������Ʒ����ϴ�Ӹɾ���ʹ��ʱ�ĵ�һ��������
�ٵ��۵⻯����ֽ����Cl2������ ��
�ڷ�Һ©������Һ�� ��
��2�������й�ʵ��������У��������� �� ��ѡ���۷֣�
A���ü�ʽ�ζ�����ȡ25.00mL��ˮ
B�������ô����������������ƻ�̼����
C���ⶨ��Һ��pHֵʱ�ø���ྻ�IJ�����պȡ��Һ������������ˮʪ�����pH��ֽ�ϣ��������ɫ���Ƚ�
D��Բ����ƿ����ƿ���ձ�����ʱ��Ӧ����ʯ������
E��ʹ������ƿ������Һʱ�����ӿ̶��߶��ݺ�������ҺŨ��ƫ��
F���ζ��õ���ƿ�͵ζ��ܶ�Ҫ����ʢ��Һ��ϴ
G������������Һʱ����������Ͳ�ڼ���һ�������ˮ�����ڽ�������������Ũ����
H����Һʱ����Һ©�����²�Һ����¿��������ϲ�Һ����Ͽڵ���
��3��±�ص���F2��Cl2��Br2��I2���۷е��ɸߵ�������Ϊ �������� ��
±����HF��HCl��HBr��HI�У��е����Ϊ �����Ϊ ����ɵ�ԭ��Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
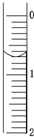 ��ʹ������к͵ζ����ⶨ���۰״���������g/100mL����
��ʹ������к͵ζ����ⶨ���۰״���������g/100mL����| �ζ����� ʵ�����ݣ�mL�� |
1 | 2 | 3 | 4 |
| V����Ʒ�� | 20.00 | 20.00 | 20.00 | 20.00 |
| V��NaOH�������ģ� | 15.95 | 15.00 | 15.05 | 14.95 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
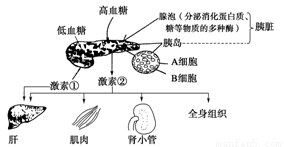
���������������������⣬��ͼ��ʾ�ȵ������ȸ�Ѫ�����ڵ��������Ǵ�л�е����ϵ����Ѫ��Ũ�Ƚ���ʱ�����آٷ��������ӣ����ͼ�����ش�
��1�������˷���ѪҺ����������ļ�����ͼ�е�________________��
��2�����آڵ����ý����������Ӱ�켤�آڵķ��ڣ��õ��ڷ�ʽ��Ϊ_____________
___________________��
��3��ͼ�еļ��آ�����_____________ϸ�����ڵġ�������_____________�����뼤�آپ�����ͬ����Ч����
��4��������ͼͨ����ĥ���ٵķ�����ü��آڣ���δ��óɹ�����ͼ��֪ԭ����_____
______________________________��
��5��Ϊ��̽���������ڼ�״�ټ��ط��ڵĵ��ڻ�����ijͬѧ���������ʵ�飺
֪ʶ�������Ǻϳɼ�״�ټ��ص�ԭ�ϡ���״���Ǻϳɡ����桢���ڼ�״�ټ��ص����١�
�������裺
��һ����ȡ��������15ֻ��ע�䲿λ�����ֱ��ÿֻ��ע�������ķ����Ե���Һ��
�ڶ�����ÿ��һ��ʱ���÷����Բ����Ƿֱ�ⶨÿֻ���Ӽ�״���е�ķ���������¼������ƽ��ֵ��
��������3���15ֻʵ�����������ΪA��B��C���顣
���IJ�����A��ע��һ�����������Եļ�״�ټ�����Һ���� B��ע������������ԵĴټ�״�ټ�����Һ����C��ע�������������ˮ��
���岽��ÿ��һ��ʱ�䣬�ֱ�ⶨ�������Ӽ�״���е�ķ���������¼������ƽ��ֵ��
�ش��������⣺
��ָ������ʵ�鷽�������һ���е��������Ͻ�֮����__________________________
_________________________________________________��
�����ʵ�鲽���һ��������ʵʩ����ʵ�飬����Ԥ������ʵ������
A��B��C�������Ӽ�״���е�ķ�����ƽ��ֵ�Ӹߵ������е�˳����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ�����и�������������ۣ���ѧ���� ���ͣ��ۺ���
���������������������⣬��ͼ��ʾ�ȵ������ȸ�Ѫ�����ڵ��������Ǵ�л�е����ϵ����Ѫ��Ũ�Ƚ���ʱ�����آٷ��������ӣ����ͼ�����ش�
��1�������˷���ѪҺ����������ļ�����ͼ�е�________________��
��2�����آڵ����ý����������Ӱ�켤�آڵķ��ڣ��õ��ڷ�ʽ��Ϊ_____________
___________________��
��3��ͼ�еļ��آ�����_____________ϸ�����ڵġ�������_____________�����뼤�آپ�����ͬ����Ч����
��4��������ͼͨ����ĥ���ٵķ�����ü��آڣ���δ��óɹ�����ͼ��֪ԭ����_____
______________________________��
��5��Ϊ��̽���������ڼ�״�ټ��ط��ڵĵ��ڻ�����ijͬѧ���������ʵ�飺
֪ʶ�������Ǻϳɼ�״�ټ��ص�ԭ�ϡ���״���Ǻϳɡ����桢���ڼ�״�ټ��ص����١�
�������裺
��һ����ȡ��������15ֻ��ע�䲿λ�����ֱ��ÿֻ��ע�������ķ����Ե���Һ��
�ڶ�����ÿ��һ��ʱ���÷����Բ����Ƿֱ�ⶨÿֻ���Ӽ�״���е�ķ���������¼������ƽ��ֵ��
��������3���15ֻʵ�����������ΪA��B��C���顣
���IJ�����A��ע��һ�����������Եļ�״�ټ�����Һ���� B��ע������������ԵĴټ�״�ټ�����Һ����C��ע�������������ˮ��
���岽��ÿ��һ��ʱ�䣬�ֱ�ⶨ�������Ӽ�״���е�ķ���������¼������ƽ��ֵ��
�ش��������⣺
��ָ������ʵ�鷽�������һ���е��������Ͻ�֮����__________________________
_________________________________________________��
�����ʵ�鲽���һ��������ʵʩ����ʵ�飬����Ԥ������ʵ������
A��B��C�������Ӽ�״���е�ķ�����ƽ��ֵ�Ӹߵ������е�˳����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС��������ѧ֪ʶ��������ʵ���о�����ش��������⣺
(1) ���ø�����طֽ������������ⶨ������ص�����������ʵ��ֹͣ������ͼI��ʾ(���ܳ��ڸ���Һ��)��
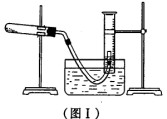
�ٲ����ռ������������������ļ������裺a��������Ͳ����Һ��߶�ʹ֮��ͬ��
b��ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£�c����ȡ��Ͳ��������������������������
ȷ˳���ǣ�___________(����д�������)��
�ڲ����ռ����������ʱ�����ʹ��Ͳ����Һ��ĸ߶���ͬ?____________��
(2) Ϊ�˵õ��������̣�ʵ����Ͻ�ͼI�е��Թ���ȴ��ʣ���ҩƷ������
���м�ˮ�ܽ⣬�پ����ˡ�ϴ�Ӻ���õ������Ķ������̡������ˡ���ʹ�õIJ�����
����_____________________��
(3) ͼ����ʵ������ȡTiCl4����װ�á����Ȼ�������ɫҺ�壬�е�Ϊ1360C������
��ˮ�⣬�������е�ˮ���������������̡�(TiCl4+H2O=TiOCl2+2HCl��)����
6500C��8500C�£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�CO���塣
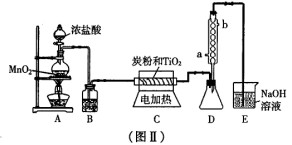
��Aװ���з�Ӧ�����ӷ���ʽ______________________________
��Bװ���е��Լ�����������______________________________
��Dװ��������ˮ�ķ���Ϊ_______________��_______________����
����ָ����װ�õ�ȱ�ݺͲ���֮��
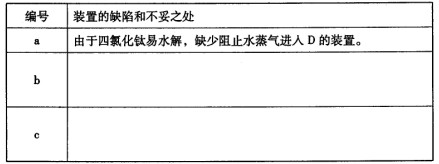
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о���ѧϰС��������ѧ֪ʶ��������ʵ���о�����ش��������⣺
(1) ���ø�����طֽ������������ⶨ������ص�����������ʵ��ֹͣ������ͼI��ʾ(���ܳ��ڸ���Һ��)��
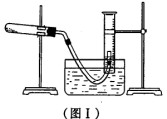
�ٲ����ռ������������������ļ������裺a��������Ͳ����Һ��߶�ʹ֮��ͬ��
b��ʹ�Թܺ���Ͳ�ڵ����嶼��ȴ�����£�c����ȡ��Ͳ��������������������������
ȷ˳���ǣ�___________(����д�������)��
�ڲ����ռ����������ʱ�����ʹ��Ͳ����Һ��ĸ߶���ͬ?____________��
(2) Ϊ�˵õ��������̣�ʵ����Ͻ�ͼI�е��Թ���ȴ��ʣ���ҩƷ������
���м�ˮ�ܽ⣬�پ����ˡ�ϴ�Ӻ���õ������Ķ������̡������ˡ���ʹ�õIJ�����
����_____________________��
(3) ͼ����ʵ������ȡTiCl4����װ�á����Ȼ�������ɫҺ�壬�е�Ϊ1360C������
��ˮ�⣬�������е�ˮ���������������̡�(TiCl4+H2O=TiOCl2+2HCl��)����
6500C��8500C�£�������ͨ���������Ѻ�̿�۵Ļ����ɵõ����Ȼ��Ѻ�CO���塣

��Aװ���з�Ӧ�����ӷ���ʽ______________________________
��Bװ���е��Լ�����������______________________________
��Dװ��������ˮ�ķ���Ϊ_______________��_______________����
����ָ����װ�õ�ȱ�ݺͲ���֮��
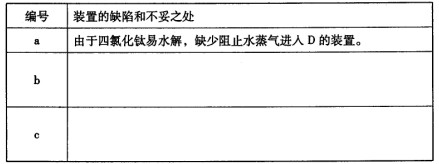
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com