
ЁОЬтФПЁПдФЖСЯТСажЊЪЖЃЌЛиД№ЃЈ1ЃЉЁЂЃЈ2ЃЉЮЪЬтЁЃ
Ђё.НсЙЙжаУЛгаЛЗзДЕФЭщЬўгжУћПЊСДЭщЬўЃЌЦфЗжзгЪНЪЧCnH2n+2ЃЈnЮЊе§ећЪ§ЃЉЃЌЗжзгжаУПМѕЩй2ИіЬМЧтМќЃЌБиШЛЭЌЪБЛсдіМг1ИіЬМЬММќЁЃЫќПЩФмЪЧжиМќЃЈЫЋМќЛђШўМќЃЉЃЌвВПЩФмЪЧСЌНгЛЗзДЬўЁЃЖМГЦЮЊдіМгСЫвЛИіВЛБЅКЭЖШЃЈгУЯЃРАзжФИІИБэЪОЁЃгжГЦЁАШБЧтжИЪ§ЁБЃЉЁЃШчБћЯЉЕФІИ=1ЃЛБНЕФІИ=4ЁЃ
Ђђ.дкгаЛњЮяЗжзгжаЃЌЕБФГИіЬМдзгЫљСЌЕФЪЧ4ИіВЛЭЌЕФдзгЛђдзгЭХЃЌИУЬМдзгНаВЛЖдГЦЬМдзгЃЌЭЈГЃгУаЧКХБъГіЃЈ*CЃЉЃЌВЂОпгаЬиЪтЕФЙтбЇЛюадЁЃ
ЃЈ1ЃЉОнБЈЕРЃЌзюНќгаШЫЪзДЮШЫЙЄКЯГЩСЫвЛжжгаПЙАЉЛюадЕФЛЏКЯЮяDepudecinетжжЮяжЪдјДгецОњРяЗжРыГіРДЃЌЦфНсЙЙШчЭМЫљЪОЃК
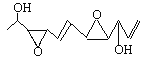
ЂйЪдаДГіетжжЛЏКЯЮяЕФЗжзгЪН________________ЃЛИУЛЏКЯЮяЕФІИЮЊ____________ЁЃ
ЂкетИіЗжзгЕФНсЙЙРяга__________ИіВЛЖдГЦЬМдзгЃПЧыдкЬтФПжаИјГіЕФНсЙЙМђЪОРягУ*АбЫќУЧБэЪОГіРДЁЃ
ЃЈ2ЃЉаТНќКЯГЩЕФФГгаЛњЮяОВтЖЈОпгаЙтбЇЛюадЃЌЦфНсЙЙЪНШчЭМЫљЪОЃК
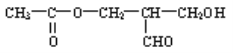
ШєвЊЪЙИУгаЛњЮяВЛдйОпгаЙтбЇЛюадЃЌФуПЩВЩгУФФаЉЛЏбЇЗНЗЈЃПЧыЗжБ№СаГіЃК__________________ЁЂ__________________ЁЂ__________________ЃЈаДГіШ§жжЃЉЁЃ
ЁОД№АИЁПC11H16O446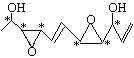 МгГЩЯћШЅЛђЫЎНтЁЃ
МгГЩЯћШЅЛђЫЎНтЁЃ
ЁОНтЮіЁП
ЃЈ1ЃЉЂйИљОнгаЛњЮяЬМдзгГЩМќЬиЕуЃЌИУгаЛњЮяЕФЗжзгЪНЮЊC11H16O4ЃЌЩйСНИіHдзгЮЊвЛИіВЛБЅКЭЖШЃЌЕУГіЃКвЛИіЛЗЮЊвЛИіВЛБЅКЭЖШЃЌвЛИіЫЋМќЮЊвЛИіВЛБЅКЭЖШЃЌИљОнНсЙЙМђЪНЃЌИУгаЛњЮяЕФВЛБЅКЭЖШЮЊ4ЃЛЂкИљОнЬтжааХЯЂЃЌЪжадЬМдзгЮЊВЛЖдГЦЬМдзгЃЌЪжадЬМдзгСЌга4ИіВЛЭЌЕФдзгЛђдзгЭХЃЌвђДЫга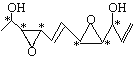 ЃЌга6ИіВЛЖдГЦЬМдзгЃЛЃЈ2ЃЉИљОнЪжадЬМдзгЕФЖЈвхЃЌСЌгаЃCH2OHКЭЃCHOЕФЁАCHЁБжаЕФCЮЊЪжадЬМдзгЃЌвђДЫЯћГ§ЙтбЇЛюадЃЌашвЊНјаагыH2ЕФМгГЩЗДгІЁЂЗЂЩњЫЎНтЗДгІЁЂбѕЛЏЗДгІЁЂЯћШЅЗДгІЁЃ
ЃЌга6ИіВЛЖдГЦЬМдзгЃЛЃЈ2ЃЉИљОнЪжадЬМдзгЕФЖЈвхЃЌСЌгаЃCH2OHКЭЃCHOЕФЁАCHЁБжаЕФCЮЊЪжадЬМдзгЃЌвђДЫЯћГ§ЙтбЇЛюадЃЌашвЊНјаагыH2ЕФМгГЩЗДгІЁЂЗЂЩњЫЎНтЗДгІЁЂбѕЛЏЗДгІЁЂЯћШЅЗДгІЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЗБћЭщПЩзїЮЊШЋЩэТщзэМСЃЌЛЗвбЭщЪЧживЊЕФгаЛњШмМСЃЌЯТУцЪЧВПЗжЛЗЭщЬўМАЭщЬўбмЩњЮяЕФ НсЙЙМђЪНЁЂМќЯпЪНКЭФГаЉгаЛњЛЏКЯЮяЕФЗДгІЪНЃЈЦфжа PtЁЂNi ЪЧДпЛЏМСЃЉЁЃ



ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛЗЭщЬўгы____________ЪЧЭЌЗжвьЙЙЬхЁЃ
ЃЈ2ЃЉДгЗДгІЂйЁЋЂлПЩвдПДГіЃЌзюШнвзЗЂЩњПЊЛЗМгГЩЗДгІЕФЛЗЭщЬўЪЧ____________ЃЈЬюУћГЦЃЉЁЃХаЖЯвР ОнЮЊ____________ЁЃ
ЃЈ3ЃЉЛЗЭщЬўЛЙПЩвдгыТБЫиЕЅжЪЃЌТБЛЏЧтЗЂЩњРрЫЦЕФПЊЛЗМгГЩЗДгІЃЌШчЛЗЖЁЭщгы HBr дквЛЖЈЬѕМўЯТЗД гІЃЌЦфЛЏбЇЗНГЬЪНЮЊ____________ЃЈВЛашзЂУїЗДгІЬѕМўЃЉЁЃ
ЃЈ4ЃЉаДГіМјБ№ЛЗБћЭщКЭБћЯЉЕФвЛжжЗНЗЈЃЎЪдМС____________ЃЛЯжЯѓгыНсТл____________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЪЕбщВЛФмДяЕНЪЕбщФПЕФЛђепЪЕбщВйзїВЛе§ШЗЕФЪЧ (ЁЁЁЁ)
A. ЖЈШн B. БШНЯСђЁЂЬМЁЂЙшШ§жждЊЫиЕФЗЧН№Ъєад
B. БШНЯСђЁЂЬМЁЂЙшШ§жждЊЫиЕФЗЧН№Ъєад C. ЮВЦјДІРэ
C. ЮВЦјДІРэ D. ВтЖЈЛЦЭжааПЕФКЌСП
D. ВтЖЈЛЦЭжааПЕФКЌСП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП(1)2017ФъжаПЦдКФГбаОПЭХЖгЭЈЙ§ЩшМЦвЛжжаТаЭNa-Fe3O4/HZSM-5ЖрЙІФмИДКЯДпЛЏМСЃЌГЩЙІЪЕЯжСЫCO2жБНгМгЧтжЦШЁаСЭщжЕЦћгЭЃЌИУбаОПГЩЙћБЛЦРМлЮЊЁАCO2ДпЛЏзЊЛЏСьгђЕФЭЛЦЦадНјеЙЁБЁЃ
вбжЊЃКH2(g)+1/2O2(g)=H2O(l) ІЄH1 = ЃaKJ/mol
C8H18(1)+25/2O2(g)=8CO2(g)+9H2O(1) ІЄH2= ЃbKJ/mol
ЪдаДГі25ЁцЁЂ101kPaЬѕМўЯТЃЌCO2гыH2ЗДгІЩњГЩЦћгЭ(вдC8H18БэЪО)ЕФШШЛЏбЇЗНГЬЪН_________________________________ЁЃ
(2)РћгУCO2МАH2ЮЊдСЯЃЌдкКЯЪЪЕФДпЛЏМС(ШчCu/ZnOДпЛЏМС)зїгУЯТЃЌвВПЩКЯГЩCH3OHЃЌЩцМАЕФЗДгІгаЃК
МзЃКCO2(g)+3H2(g) ![]() CH3OH(g)+H2O(g) ЁїH= ЁЊ 53.7kJЁЄmol-1 ЦНКтГЃЪ§K1
CH3OH(g)+H2O(g) ЁїH= ЁЊ 53.7kJЁЄmol-1 ЦНКтГЃЪ§K1
ввЃКCO2(g)+H2(g) ![]() CO(g)+H2O(g) ЁїH= + 41.2kJЁЄmol-1 ЦНКтГЃЪ§K2
CO(g)+H2O(g) ЁїH= + 41.2kJЁЄmol-1 ЦНКтГЃЪ§K2
ЂйCO(g)+2H2(g) ![]() CH3OH(g)ЕФЦНКтГЃЪ§K=______(гУКЌK1ЁЂK2ЕФБэДяЪНБэЪО)ЃЌИУЗДгІЁїH_____0(ЬюЁАДѓгкЁБЛђЁАаЁгкЁБ)ЁЃ
CH3OH(g)ЕФЦНКтГЃЪ§K=______(гУКЌK1ЁЂK2ЕФБэДяЪНБэЪО)ЃЌИУЗДгІЁїH_____0(ЬюЁАДѓгкЁБЛђЁАаЁгкЁБ)ЁЃ
ЂкЬсИпCO2зЊЛЏЮЊCH3OHЦНКтзЊЛЏТЪЕФДыЪЉга___________(ЬюаДСНЯю)ЁЃ
ЂлДпЛЏМСКЭЗДгІЬхЯЕЕФЙиЯЕОЭЯёЫјКЭдПГзЕФЙиЯЕвЛбљЃЌОпгаИпЖШЕФбЁдёадЁЃЯТСаЫФзщЪЕбщЃЌПижЦCO2КЭH2ГѕЪМЭЖСЯБШОљЮЊ1ЃК2.2ЃЌОЙ§ЯрЭЌЗДгІЪБМф(t1min)ЁЃ
ЮТЖШ(K) | ДпЛЏМС | CO2зЊЛЏТЪ(%) | МзДМбЁдёад(%) | злКЯбЁЯю |
543 | Cu/ZnOФЩУзАєВФСЯ | 12.3 | 42.3 | A |
543 | Cu/ZnOФЩУзЦЌВФСЯ | 11.9 | 72.7 | B |
553 | Cu/ZnOФЩУзАєВФСЯ | 15.3 | 39.1 | C |
553 | Cu/ZnOФЩУзЦЌВФСЯ | 12.0 | 70.6 | D |
гЩБэИёжаЕФЪ§ОнПЩжЊЃЌЯрЭЌЮТЖШЯТВЛЭЌЕФДпЛЏМСЖдCO2ЕФзЊЛЏЮЊCH3OHЕФбЁдёадгаЯджјгАЯьЃЌИљОнЩЯБэЫљИјЪ§ОнНсКЯЗДгІдРэЃЌЫљЕУзюгХбЁЯюЮЊ___________(ЬюзжФИЗћКХ)ЁЃ
(3)вдCOЁЂH2ЮЊдСЯКЯГЩМзДМЕФЗДгІЮЊЃКCO(g)+2H2(g)![]() CH3OH(g)ЁЃдкЬхЛ§ОљЮЊ2LЕФШ§ИіКуШнУмБеШнЦїЂёЁЂЂђЁЂЂѓжаЃЌЗжБ№ЖМГфШы1molCOКЭ2molH2ЃЌШ§ИіШнЦїЕФЗДгІЮТЖШЗжБ№ЮЊT1ЁЂT2ЁЂT3ЧвКуЖЈВЛБфЁЃЯТЭМЮЊШ§ИіШнЦїжаЕФЗДгІОљНјааЕН5minЪБH2ЕФЬхЛ§ЗжЪ§ЪОвтЭМЃЌЦфжагавЛИіШнЦїЗДгІвЛЖЈДяЕНЦНКтзДЬЌЁЃ
CH3OH(g)ЁЃдкЬхЛ§ОљЮЊ2LЕФШ§ИіКуШнУмБеШнЦїЂёЁЂЂђЁЂЂѓжаЃЌЗжБ№ЖМГфШы1molCOКЭ2molH2ЃЌШ§ИіШнЦїЕФЗДгІЮТЖШЗжБ№ЮЊT1ЁЂT2ЁЂT3ЧвКуЖЈВЛБфЁЃЯТЭМЮЊШ§ИіШнЦїжаЕФЗДгІОљНјааЕН5minЪБH2ЕФЬхЛ§ЗжЪ§ЪОвтЭМЃЌЦфжагавЛИіШнЦїЗДгІвЛЖЈДяЕНЦНКтзДЬЌЁЃ
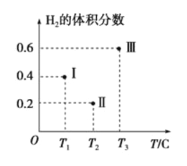
Ђй0ЁЋ5minЪБМфФкШнЦїЂђжагУCH3OHБэЪОЕФЛЏбЇЗДгІЫйТЪЮЊ_________________ЁЃ
ЂкШ§ИіШнЦїжавЛЖЈДяЕНЦНКтзДЬЌЕФЪЧШнЦї________(ЬюаДШнЦїДњКХ)ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМжаКсзјБъБэЪОЭъШЋШМЩеЪБКФгУПЩШМЦјЬхX(X=AЁЂBЁЂC)ЕФЮяжЪЕФСПn(x)ЁЃзнзјБъБэЪОЯћКФO2ЕФЮяжЪЕФСПn(O2)ЃЌAЁЂBЪЧСНжжПЩШМЦјЬхЃЌCЪЧAКЭBЕФЛьКЯЦјЬхЃЌдђCжаn(A)ЁУn(B)ЮЊ ЃЈ ЃЉ

A. 2ЁУ1 B. 1ЁУ2 C. 1ЁУ1 D. ШЮвтБШ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПОнБЈЕРЃЌСзЫсЖўЧтМиЃЈKH2PO4ЃЉДѓОЇЬхвбгІгУгкЮвЙњбажЦЕФОоаЭМЄЙтЦїЁАЩёЙтЖўКХЁБжаЁЃРћгУЗњСзЛвЪЏЃЈЛЏбЇЪНЮЊCa5P3FO12)жЦБИСзЫсЖўЧтМиЕФЙЄвеСїГЬШчЯТЭМЫљЪОЃЈВПЗжСїГЬВНжшвбЪЁТдЃЉЃК

вбжЊнЭШЁЕФжївЊЗДгІдРэЃКKCl+H3PO4![]() KH2PO4+HClЃЛЦфжаЃЌЗДгІВњЩњЕФHClвзШмгкгаЛњнЭШЁМСЁЃ
KH2PO4+HClЃЛЦфжаЃЌЗДгІВњЩњЕФHClвзШмгкгаЛњнЭШЁМСЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉСїГЬжаНЋЗњСзЛвЪЏЗлЫщЕФФПЕФЪЧ__________________________________ЁЃ
ЃЈ2ЃЉВЛФмЪЙгУЖўбѕЛЏЙшЬеДЩВФжЪЕФЗаЬкВлЕФжївЊдвђЪЧ___________________ЃЈгУЛЏбЇЗНГЬЪНБэЪОЃЉЁЃ
ЃЈ3ЃЉИБВњЦЗNЕФЛЏбЇЪНЪЧ____________ЃЛдкЕУЕНKH2PO4ОЇЬхЕФвЛЯЕСаВйзїЂѓЃЌЦфжївЊАќРЈ______________________________ЁЂЙ§ТЫЁЂЯДЕгЁЂИЩдяЕШЁЃ
ЃЈ4ЃЉШєгУ1000kgжЪСПЗжЪ§ЮЊ50.4%ЕФЗњСзЛвЪЏЃЈЛЏбЇЪНЮЊCa5P3FO12)РДжЦШЁСзЫсЖўЧтМиОЇЬхЃЌЦфВњТЪЮЊ80%ЃЌдђРэТлЩЯПЩЩњВњKH2PO4ЕФжЪСПЮЊ_______kgЁЃ
ЃЈ5ЃЉЕчНтЗЈжЦБИKH2PO4ЕФзАжУШчЭМЫљЪОЃЎИУЕчНтзАжУжаЃЌa ЧјЪєгк_______ЧјЃЈЬюЁАбєМЋЁБЛђЁАвѕМЋЁБЃЉЃЌвѕМЋЧјЕФЕчМЋЗДгІЪНЪЧ______________________________________ЁЃ

ЃЈ6ЃЉЙЄвЕЩЯЛЙПЩвдгУЗњСзЛвЪЏгыНЙЬПЁЂЪЏгЂЩАЛьКЯЃЌдкЕчТЏжаМгШШЕН1500ЁцЩњГЩАзСзЃЌЭЌЪБвнГіSiF4КЭCO,ИУЗДгІЕФЛЏбЇЗНГЬЪНЮЊ________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМЫљЪОЮЊШЫЬхдкФГЯюЩњРэЙ§ГЬжаЫљЗЂЩњЕФЛЏбЇЗДгІЪОвтЭМЁЃ

ЃЈ1ЃЉЭМжаБъгазжФИЕФЮяжЪжаЃЌ_____ДњБэУИЃЌОјДѓВПЗжУИЕФЛЏбЇБОжЪЪЧ___________ЃЌЛљБОзщГЩЕЅЮЛЪЧ__________ЁЃ
ЃЈ2ЃЉШчЙћBДњБэесЬЧЃЌдђCКЭDИїДњБэ______________ЁЂ________________ЁЃ
ЃЈ3ЃЉЯТСаЙигкЦЯЬбЬЧгыесЬЧЯрБШНЯЕФЫЕЗЈжаДэЮѓЕФЪЧ___________________ЁЃ
AЃЎЫќУЧЕФЗжзгЪНВЛЭЌЃЌЕЋЛЏбЇдЊЫизщГЩЯрЭЌ
BЃЎесЬЧФмЫЎНтЃЌЦЯЬбЬЧШДВЛФм
CЃЎЫќУЧЪЧЭЌЗжвьЙЙЬх
DЃЎЦЯЬбЬЧЪЧЕЅЬЧЁЂесЬЧЪЧЖўЬЧ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЖдЛЏбЇПЦбЇЕФгІгУа№ЪіВЛе§ШЗЕФЪЧЃЈЁЁЁЁЃЉ
A.ЛЏбЇПЦбЇНЋМЬајЭЦЖЏВФСЯПЦбЇЕФЗЂеЙ
B.ЛЏбЇПЦбЇНЋЮЊЛЗОГЮЪЬтЕФНтОіЬсЙЉгаСІЕФБЃеЯ
C.ЛЏбЇПЦбЇдкСЫНтМВВЁЕФВЁРэЗНУцЮоФмЮЊСІ
D.ЛЏбЇПЦбЇЪЙШЫРрФмЙЛИќКУЕиРћгУФмдДКЭзЪдД
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаЙ§ГЬБиаыМгШыЛЙдМСВХФмЪЕЯжЕФЪЧЃЈ ЃЉ
A.Cl2ЁњCl-B.Cl-ЁњCl2C.Cu2ЃЋЁњCuD.CO32-ЁњCO2
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com