
����Ŀ����1 mol N2�� 3 mol H2��������ɱ�ĺ����ܱ�������,��380 ���·�����Ӧ��N2(g)+3H2(g)![]() 2NH3(g)��ƽ��ʱ,��ϵ�а����������
2NH3(g)��ƽ��ʱ,��ϵ�а����������![]() (NH3)��ѹǿ�仯��������±�:
(NH3)��ѹǿ�仯��������±�:
ѹǿ/MPa | 10 | 20 | 30 | 40 |
| 0.30 | 0.45 | 0.54 | 0.60 |
����˵����ȷ����(����)
A.10 MPaʱ,H2��ת����Ϊ75%
B.20 MPaʱ,NH3�����ʵ���Ũ����10 MPaʱ��1.5��
C.40 MPaʱ,�����������ΪV L,��ƽ�ⳣ��K=![]()
D.30 MPaʱ,���������г����������,��ƽ��������Ӧ�����ƶ�
���𰸡�C
��������
A.10 MPaʱ��
N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
��ʼ�� 1 mol 3 mol 0
�仯�� x 3x 2x
ƽ���� 1-x 3-3x 2x
��![]()
x=![]() ��H2��ת����Ϊ
��H2��ת����Ϊ![]() ��A����
��A����
B. 20 MPaʱ��
N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
��ʼ�� 1 mol 3 mol 0
�仯�� y 3y 2y
ƽ���� 1-y 3-3y 2y
��![]()
y=![]() ��NH3�����ʵ���Ũ����10 MPaʱ��
��NH3�����ʵ���Ũ����10 MPaʱ��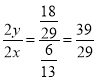 ��1.5����B����
��1.5����B����
C. 40 MPaʱ��
N2(g)+3H2(g)![]() 2NH3(g)
2NH3(g)
��ʼ�� 1 mol 3 mol 0
�仯�� z 3z 2z
ƽ���� 1-z 3-3z 2z
��![]()
z=![]() ��K=
��K=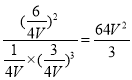 ��C��ȷ��
��C��ȷ��
D. 30 MPaʱ�����������г���������壬�������������Ӧ����������Ũ��ͬ�ȳ̶ȼ�С����ƽ�����淴Ӧ�����ƶ���D����
��ѡC��


 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ݱ�������ѧ���Ѿ����Ƴ���������IJ��ϡ���̼ĤƬ������ֻ��һ��ͷ���Ķ�ʮ���֮һ����ͼ��ʾ������̼ĤƬ��״��䳲������̼ԭ�ӹ��ɵ������ε�Ԫ������չ���ɣ������й�̼ĤƬ��˵���У���ȷ���ǣ� ��
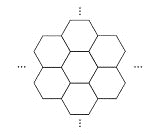
A. ̼ĤƬ����һ�����͵Ļ�����
B. ̼ĤƬ��ʯī�Ľṹ��ͬ
C. ̼ĤƬ��C60��Ϊͬ��������
D. ̼ĤƬ����������ȫȼ�յIJ����̼����������ȫȼ�յIJ��ﲻͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������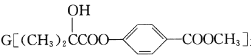 ��һ��ҽҩ�м��壬����һ�ֺϳ�·�����£�
��һ��ҽҩ�м��壬����һ�ֺϳ�·�����£�
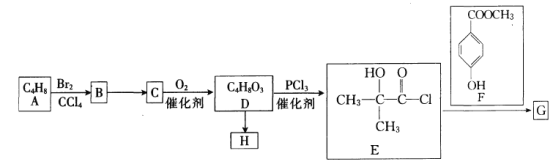
��֪��![]() ����ش��������⣺
����ش��������⣺
(1)A�Ļ�ѧ������______________��G�ķ���ʽΪ____________________��
(2)��B����C�ķ�Ӧ����Ϊ____________����E��F����G�ķ�Ӧ������___________��
(3)D��Ũ����/���������»ᷴӦ����һ�ֺ���Ԫ���Ļ�����û�����Ľṹ��ʽΪ______________��
(4)H��һ�ָ߾�����D����H�Ļ�ѧ����ʽΪ__________________________________��
(5)��������Ҫ���F��ͬ���칹�干��___________�֡�
�ٱ�����������ȡ���������ܷ���������Ӧ����1mol����������2molNa2CO3��Ӧ��
��д��������������Ũ��ˮ��Ӧ�����ò���ı����ϲ�������ԭ�ӵ�F��ͬ���칹��ṹ��ʽ��_______________________(��дһ��)��
(6)��֪���ǻ����������ᷢ��������Ӧ��д���Ա��ӡ��ױ�Ϊԭ���Ʊ������ᱽ��![]() �ĺϳ�·��(�������Լ���ѡ)��____________��
�ĺϳ�·��(�������Լ���ѡ)��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ������Һ���Ƿ��г��������������ӣ���������ͼ��ʾ��ʵ����������м�������в�����������ʹʪ��ĺ�ɫʯ����ֽ�������ɸ�ʵ���ܵõ�����ȷ������
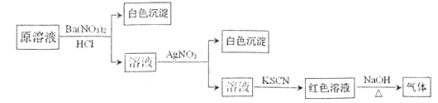
A.ԭ��Һ��һ������![]() ����B.ԭ��Һ��һ������
����B.ԭ��Һ��һ������![]()
C.ԭ��Һ��һ������Cl-����D.ԭ��Һ��һ������Fe3+����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����![]() �Ӻ�ˮ�õ��Ĵ�ʳ��ˮ�г���
�Ӻ�ˮ�õ��Ĵ�ʳ��ˮ�г���![]() ��
��![]() ��
��![]() ����Ҫ�����ᴿ
����Ҫ�����ᴿ![]() ���к�����
�������![]() ��
��![]() �Ĵ�ʳ��ˮ���ڳ�ȥ���������ɳ֮��Ҫ��4���Լ�
�Ĵ�ʳ��ˮ���ڳ�ȥ���������ɳ֮��Ҫ��4���Լ�![]() ���ᡢ
���ᡢ![]() ��
��![]() ��
��![]() ����ȥʳ��ˮ��
����ȥʳ��ˮ��![]() ��
��![]() ��
��![]() �ᴿʱ�IJ�������ͼ����Լ��������ͼ1��
�ᴿʱ�IJ�������ͼ����Լ��������ͼ1��
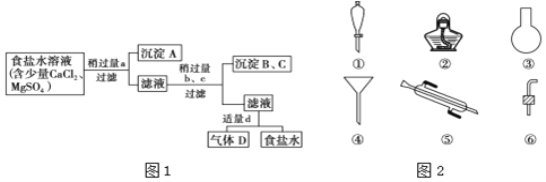
ͼ1��a��b��c��d�ֱ��ʾ����4���Լ��е�һ�֣��Իش�
![]() ����A��������______��
����A��������______��
![]() �Լ�d��______���ж��Լ�d�����ķ�����______��
�Լ�d��______���ж��Լ�d�����ķ�����______��
![]() ����b��c����Һ�з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽΪ______��
����b��c����Һ�з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽΪ______��
��![]() �������������ʾ����������Եõ���ˮ�����ӵ�ˮ����ȡ�⣬��Ҫͼ2�����е�______
�������������ʾ����������Եõ���ˮ�����ӵ�ˮ����ȡ�⣬��Ҫͼ2�����е�______![]() �����
�����![]() ����õ�ˮ�м������Ȼ�̼����ȡ�ⵥ�ʵ�ʵ���������______��
����õ�ˮ�м������Ȼ�̼����ȡ�ⵥ�ʵ�ʵ���������______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ơ�������������Դ�������Ⱦ�Ǹ��ƴ�����������Ҫ�������ش����������������йص����⣺
��.(1)�ڴ��������£�����ɻ�ԭ��������Ӷ��ﵽ��������������Ⱦ��Ŀ�ġ���֪����CH4(g)��4NO2(g)= 4NO(g)��CO2(g)��2H2O(g)����H����574 kJ��mol��1����4NO2(g)��2N2(g)= 8NO(g)����H����586 kJ��mol��1����CH4(g)��4NO(g)= 2N2(g)��CO2(g)��2H2O(g)����H��________ kJ��mol��1��
(2)��ҵ�����еĵ����������NH3����ԭ����Ӧԭ����ͼ��ʾ��

����XΪһ���������壬��NH3����ԭ��������Ļ�ѧ����ʽΪ______��
(3)��NH3����ԭ�����еĵ�������ʱ����![]() ��x����Fe������ʱ����NH3����������£���ͬxֵ��Ӧ���ѵ�����(����ԭ�ĵ�������İٷ���)��ͬ���ڲ�ͬ�¶������ϵ��ͼ��ʾ����x��_____ʱ���ѵ�Ч����ѣ���ѵ��ѵ��¶���____ �档
��x����Fe������ʱ����NH3����������£���ͬxֵ��Ӧ���ѵ�����(����ԭ�ĵ�������İٷ���)��ͬ���ڲ�ͬ�¶������ϵ��ͼ��ʾ����x��_____ʱ���ѵ�Ч����ѣ���ѵ��ѵ��¶���____ �档
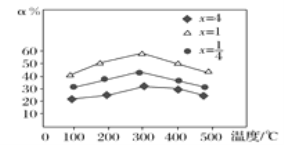
��.(4)�״���һ����ɫȼ�ϣ��״��Ĺ�ҵ�ϳɷ����϶࣬��CO(g)��2H2(g)![]() CH3OH(g)����2 L�����ܱ������г���1 mol CO��2 mol H2���ԭ��������ַ�Ӧ�ﵽƽ�⣬���ƽ��ʱ�������CH3OH�����������ѹǿ���¶ȵĹ�ϵ��ͼ��ʾ��
CH3OH(g)����2 L�����ܱ������г���1 mol CO��2 mol H2���ԭ��������ַ�Ӧ�ﵽƽ�⣬���ƽ��ʱ�������CH3OH�����������ѹǿ���¶ȵĹ�ϵ��ͼ��ʾ��
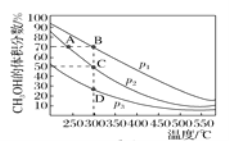
��ͼ��ѹǿp1��p2��p3�Ĵ�С��ϵ��_______��
��C��ƽ�ⳣ��K��___��A��B��D�����ƽ�ⳣ��K(�ֱ���KA��KB��KD��ʾ)�Ĵ�С��ϵ��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�ϳɰ���ӦΪ��N2��g��+3H2��g��![]() 2NH3��g���������о����£�
2NH3��g���������о����£�
��1����֪H��H����Ϊ436kJ��mol��1��N��H����Ϊ391kJ��mol��1��N��N���ļ�����946kJ��mol��1����������Ӧ����H��_________________��
��2��������Ӧ��ƽ�ⳣ��K�ı���ʽΪ____________������Ӧ����ʽ��дΪNH3��g��![]() N2��g��+
N2��g��+![]() H2��g������ƽ�ⳣ��K1��____________________����K��ʾ����
H2��g������ƽ�ⳣ��K1��____________________����K��ʾ����
��3����773Kʱ���ֱ�2mol N2��6mol H2����һ���̶��ݻ�Ϊ1L���ܱ������У����ŷ�Ӧ�Ľ��У�����������n��H2����n��NH3���뷴Ӧʱ��t�Ĺ�ϵ���±���
t/min | 0 | 5 | 10 | 15 | 20 | 25 | 30 |
n��H2��/mol | 6.00 | 4.50 | 3.60 | 3.30 | 3.03 | 3.00 | 3.00 |
n��NH3��/mol | 0 | 1.00 | m | 1.80 | 1.98 | 2.00 | 2.00 |
�ٱ�����m��_______________/span>��15��25min�ڣ�v��N2����_______________��
�ڸ��¶��£�����ͬ�ݻ�����һ������Ͷ���N2��H2��NH3Ũ�Ⱦ�Ϊ3mol��L��1����ʱv��_______v��������������������������������
���ɱ��е�ʵ�����ݼ���õ���Ũ�ȡ�ʱ�����Ĺ�ϵ����ͼ�е����߱�ʾ����ʾc��N2����t��������______________�������������������������������ڴ��¶��£�����ʼ����4mol N2��12mol H2����Ӧ�մﵽƽ��ʱ����ʾc��H2������������Ӧ�ĵ�Ϊ_________________��

��4��Marnellos��Stoukides���õ�ⷨ�ϳɰ���ʵ���˳�ѹ�ϳɺ͵����ĸ�ת���ʡ��÷�����SCY�մɽ�����������SCY�մɾ��и����ӵ����ԣ��������Ǵ���H�����������ĵ缫��ӦΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ҵ�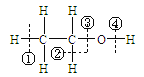 �ڻ�ѧ��Ӧ�жϼ�λ��˵��������� �� ��
�ڻ�ѧ��Ӧ�жϼ�λ��˵��������� �� ��
A. �Ҵ���Ũ������170��ʱ���ڢڢ�λ�ö��ѣ���ˮ���ӵ���ʽ��ȥ��������ȥ��Ӧ��
B. �Ҵ����Ʒ�Ӧ���ڢ�λ�ö��ѣ��Ա�����ˮ��Ӧ���Ҵ���ˮ����ʧȥ�����ӡ�
C. �Ҵ���Ũ������140��ʱ���ڢۻ��λ�ö��ѣ��÷�Ӧ����ȡ����Ӧ��
D. �Ҵ���ͭ������������Ӧ���ڢڢ�λ�ö��ѣ�����ֻ�����ǻ�ֱ��������̼ԭ��������ԭ�Ӳ��ܷ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������㷺Ӧ������ҩ�ͻ�����ҵ��ijͬѧ�����üױ���������Ӧ�Ʊ������ᡣ��Ӧԭ��:
![]() +2KMnO4
+2KMnO4![]() +KOH+2MnO2��+H2O
+KOH+2MnO2��+H2O
![]() +HCl��
+HCl��![]() +KCl
+KCl
ʵ�鷽��:һ�����ļױ���KMnO4��Һ��100�淴Ӧһ��ʱ���ֹͣ��Ӧ�����������̷����������ͻ���δ��Ӧ�ļױ���
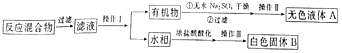
��֪:��������Է���������122���۵�122.4�棬��25���95��ʱ�ܽ�ȷֱ�Ϊ0.3g��6.9g�����������л���һ�㶼�й̶��۵㡣
��1������1Ϊ_________________��������Ϊ������������ѡ�õIJ�����������Բ����ƿ������ͷ���¶ȼơ����չܣ�ţ�ǹܣ�֮�⣬����___________������ţ�
A.���������� B.ֱ�������� C.����ƿ D.�ձ�
��2�����������ʵ�鲽������Ϊ__________�����ˡ������ʷ��ڲ���©���д�����һ��ʱ�����©����ʣ����ǰ�ɫ����B������װ�ÿ��Լӿ���������乤��ԭ����_______
��3���ⶨ��ɫ����B���۵㣬��������115�濪ʼ�ۻ��ﵽ130��ʱ�����������ۡ���ͬѧ�Ʋ��ɫ����B�DZ�����������KCl�Ļ���
�����ӻ�������ᴿ������Ӧ���еIJ�����_______________________
���ڢ��ᴿ������Ӧ�Ծ������ϴ�ӳ�ȥ������渽�����ʣ���μ���ϴ���Ѿ���ȫ:_______
�������龭�����ᴿ��Ĺ����Ƿ������Բ��õ�ʵ�鷽����______,������崿����Ӧ��������___________________________
��4�����Ȳⶨ:��ȡ1.220g��Ʒ�����100mL�״���Һ��ȡ25.00mL��Һ�ζ�������KOH�����ʵ���Ϊ2.40��10-3mol��Ʒ�б�������������Ϊ_________��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com