
����Ŀ��25��ʱ����25mL0.1mol��L��1��NaOH��Һ�У���μ���0.2mol��L��1��CH3COOH��Һ����ҺpH�ı仯������ͼ��ʾ�����з����Ľ����У�����ȷ����

A.C��ʱc(CH3COO��)��c(Na��)��c(H��)��c(OH��)
B.D��ʱc(CH3COO��)��c(CH3COOH)��2c(Na��)
C.������A��B����һ�㣬��Һ�ж��У�c(Na��)��c(CH3COO��)>c(OH-)>c(H��)
D.B������a��12��5ml
���𰸡�D
��������
A�����ݵ���غ㣬c(CH3COO��)+c(OH��)=c(Na��)+c(H��)��C����Һ�����ԣ�����c(CH3COO��)��c(Na��)��c(H��)��c(OH��)����A��ȷ��
B��D��ʱc(CH3COO��)��c(CH3COOH)��2c(Na��)�����������غ㣬��B��ȷ��
C�������뼰��������ʱ��c(OH-)>c(CH3COO��)����C����
D��a��12.5mlʱ���������ƺʹ���ǡ����ȫ��Ӧ����ʱ��Һ�ʼ��ԣ���D����
��ѡD��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���װ�(CH3NH2)��һ��һԪ�������뷽��ʽΪ��CH3NH2 + H2O ![]() CH3NH3+ + OH���������£���20.0 mL 0.10 mol/L�ļװ���Һ�еμ�VmL0.10mol/L��ϡ���ᣬ�����Һ��pH�������Ũ�ȵĹ�ϵ��ͼ��ʾ������˵���д������
CH3NH3+ + OH���������£���20.0 mL 0.10 mol/L�ļװ���Һ�еμ�VmL0.10mol/L��ϡ���ᣬ�����Һ��pH�������Ũ�ȵĹ�ϵ��ͼ��ʾ������˵���д������

A. b���Ӧ������������V<20.00mL
B. �����£�����a���֪�װ��ĵ���ƽ�ⳣ��Kb=10 -3��4
C. b����ܴ��ڹ�ϵ��c(Cl��) > c(CH3NH3+) > c(H+) = c(OH��)
D. V=20.00mLʱ����Һ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Na2S������������Ⱦ�ϡ�����ˮ���е��ؽ����ȡ�
(1)Na2S��Һ��S2-ˮ������ӷ���ʽΪ_________��
(2)����ʱ�������ؽ������ӵ�������ܶȻ��������±���
�������� | FeS | PbS | CuS | HgS |
Ksp | 6.3��10��18 | 1.0��10��28 | 6.3��10��36 | 1.6��10��52 |
�������ʵ���Ũ����ͬ��Fe2+��Pb2+��Cu2+��Hg2+�Ļ��ϡ��Һ�У���μ���Na2Sϡ��Һ�����ȳ�����������____��
����Na2S��Һ������ˮ��Pb2+��ΪʹPb2+������ȫ[c(Pb2��)��1��10-6mol/L]����Ӧ������Һ��c(S2-)��_____mol/L��
�۷�ӦCu2+(aq)+FeS(s)![]() Fe2+(aq)+CuS(s)��ƽ�ⳣ��K=_______��
Fe2+(aq)+CuS(s)��ƽ�ⳣ��K=_______��
(3)�ⶨijNa2S��NaHS�����Ʒ�����ߺ�����ʵ�鲽�����£�
����1.ȷ��ȡһ������Ʒ���ձ��У�������������ˮ�ܽ⣬ת����500mL����ƿ�ж��ݡ�
����2.ȷ��ȡ25.00mL������Һ����ƿ�У��������ػ�GG-��ʱ��������ָʾ������0.2500mol/L�������Һ�ζ���Na2S+HCl=NaHS+NaCl�����յ㣬��������24.00mL���������ټ���5mL���Լ�ȩ(NaHS+HCHO+H2O��NaOH+HSCH2OH)��3�η�ָ̪ʾ����������0.2500mol/L�������Һ�ζ�(NaOH+HCl=NaCl+H2O)���յ㣬����������34.00mL��
����ԭ�������Na2S��NaHS�����ʵ���֮��(д���������)___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������仯����������������й㷺Ӧ�á���ش��������⣺
(1)��ʵ�����У�![]() �������ۺ�______��Ӧ�Ʊ���
�������ۺ�______��Ӧ�Ʊ���![]() �������ۺ�______��Ӧ�Ʊ���
�������ۺ�______��Ӧ�Ʊ���
(2)������![]() ��Ҫ�ɷ�Ϊ
��Ҫ�ɷ�Ϊ![]() �����������ұ����������Ҫԭ�ϡ������¿ɷ�����Ӧ��
�����������ұ����������Ҫԭ�ϡ������¿ɷ�����Ӧ��
![]() ���ù���������
���ù���������![]()
![]() �μӷ�Ӧ����Ӧ������ת��______mol���ӡ�
�μӷ�Ӧ����Ӧ������ת��______mol���ӡ�
(3)![]() ��Zn������Ͷ��ε�ظ�����أ����ҺΪ����Һ���䷴ӦʽΪ��
��Zn������Ͷ��ε�ظ�����أ����ҺΪ����Һ���䷴ӦʽΪ��![]() ���ŵ�ʱ��صĸ�����ӦʽΪ______�����ʱ���Һ��pH______
���ŵ�ʱ��صĸ�����ӦʽΪ______�����ʱ���Һ��pH______![]() ��������������С������������֮һ
��������������С������������֮һ![]() ��
��
(4)ijͬѧ��ʢ��![]() ��Һ���Թ��м��뼸���ữ��
��Һ���Թ��м��뼸���ữ��![]() ��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ______��һ��ʱ�����Һ�������ݳ��֣������ȣ�����к��ɫ�������ɡ��������ݵ�ԭ����______�����ɳ�����ԭ����______
��Һ����Һ����ػ�ɫ��������Ӧ�����ӷ���ʽΪ______��һ��ʱ�����Һ�������ݳ��֣������ȣ�����к��ɫ�������ɡ��������ݵ�ԭ����______�����ɳ�����ԭ����______![]() ��ƽ���ƶ�ԭ������
��ƽ���ƶ�ԭ������![]() ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ�鲻�ܴﵽԤ��Ŀ����

A.ʵ������������ͷ�ι��е�NaOH��Һ���۲�Fe(OH)2��������ɫ
B.ʵ���������������Һ�����ɫ��ֹͣ���ȣ�������ͨ����ϵʱ���������ЧӦ
C.ʵ������ͨ���۲�����KMnO4��Һ��ɫ��ȥ��ȷ������ϩ����
D.ʵ����������һ���¶ȣ���ʯ�ͷ���Ϊ���͡����͵�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��п������Ҫ��Zn��ZnO��SiO2��Fe2+��Cd2+��Mn2+����ҵ�Ͽ�ͨ������������һ��ȥ�������Ʊ���ϸ��������п���乤���������£�
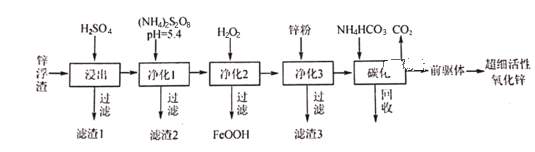
(1)����1�ijɷ�Ϊ___________��
(2)��S2O82���Ľṹʽ��ֻ����һ����OһO���Ǽ��Լ�����S�Ļ��ϼ�Ϊ___________����ҵ�ϳ��ö��Ե缫���(NH4)2SO4����(NH4)2S2O8(���������)�������缫��ӦʽΪ__________________������1��Ϊ�˽�Mn2+ת��ΪMnO2����ȥ��д���÷�Ӧ�����ӷ���ʽ��______________________��
(3)����3��Ŀ��_________________________________��
(4)̼��������Һ����Ҫ�ɷ�Ϊ___________��������ѭ��ʹ�õ�Ŀ��___________________��
(5)̼����50����У���ǰ���塱�Ļ�ѧʽΪZnCO3��2Zn(OH)2��H2O��д��̼���������ɡ�ǰ���塱�Ļ�ѧ����ʽ��__________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ӹ�ҵ�Ϸ��л��ս������ȱ�����Ⱦ��������������Դ�ۺ����á�ij��ҵ�Ϸ�����Ҫ�ɷ�ΪV2O5��VOSO4��SiO2�ȣ���ͼ�ǴӷϷ��л��շ���һ�ֹ������̣�

��1��Ϊ������������Ч�ʣ����Բ�ȡ�Ĵ�ʩ��________(������)��
��2������ԭ�������з�Ӧ�����ӷ���ʽΪ________��
��3�����������õ�NH4VO3��������Գ�������ϴ�ӣ����������ȫϴ���ķ�����________��
��4��д�����������ȷ�Ӧ�Ļ�ѧ����ʽ________��
��5����⾫��ʱ��������NaCl��CaCl2��VCl2Ϊ���Һ(����VCl2�Է�����ʽ����).�ַ�Ӧ���Դ��________��(����������������)�����������ĵ缫��ӦʽΪ________��
��6��ΪԤ������ԭ���������H2C2O4��������ⶨ�������Һ��VO2+��Ũ�ȣ�ÿ��ȡ25.00mL�������Һ����ƿ��a mol/L(NH4)2Fe(SO4)2����Һ�ͱ����ڰ���������Ϊָʾ�����еζ�(����VO2+ VO2+)�������εζ����ı�Һ�����ƽ��ΪbmL����VO2+��Ũ��Ϊ________g/L(�ú�a��b�Ĵ���ʽ��ʾ)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������Դ������̼���ŷţ�ʵʩ��̼�����ǽ��������������
(1)���д�ʩ��������Ч���ٶ�����̼�ŷŵ���________��
A.ֲ�����֣�����ɭ�֣�����ֲ��
B.�Ӵ��ú��ʯ�͵Ŀ��ɣ�������ʹ��ʯ��Һ����
C.�ƹ�ʹ�ý��ܵƺͽ��ܵ�����ʹ�ÿյ�ʱ�ļ��¶Ȳ������ù��ͣ����첻�˹���
D.��������ʱ�ಽ�к������г��������ִ�������Ϣϵͳ���������乤�߿�ʻ��
(2)CO2�ϳ�����ȼ�ϼ״�(CH3OH)��̼���ŵ��·���������ʵ�飺ij�¶�����1 L���ܱ������У���2 mol CO2��6 mol H2��������CO2(g)+3H2(g )![]() CH3OH(g)+H2O(g)���ֲ��CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ1��ʾ���ӷ�Ӧ��ʼ��ƽ��ʱCO2��ת����Ϊ_________��������ƽ����Ӧ����v(H2)=_______mol/(L��min)�����¶��µ�ƽ�ⳣ��Ϊ________��
CH3OH(g)+H2O(g)���ֲ��CO2��CH3OH(g)��Ũ����ʱ��仯����ͼ1��ʾ���ӷ�Ӧ��ʼ��ƽ��ʱCO2��ת����Ϊ_________��������ƽ����Ӧ����v(H2)=_______mol/(L��min)�����¶��µ�ƽ�ⳣ��Ϊ________��
(3)CO�ڴ����������ɼ״���CO(g)+2H2(g)![]() CH3OH(g)����֪�ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)����֪�ܱ������г���10 mol CO��20 mol H2��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
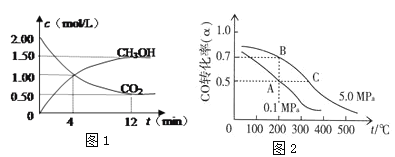
�ٸ÷�Ӧ����H_____0����S____0�� (����>��=��<��)
����A��C���㶼��ʾ�ﵽ��ƽ��״̬�����Է�Ӧ��ʼ����ƽ��״̬�����ʱ��tA ____ tC(����>��=��<��)
��A��B��C�����Ӧ��ƽ�ⳣ���ֱ�ΪKA��KB��KC����Ĵ�С��ϵ��____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��K2Cr2O7��Һ�д���ƽ�⣺Cr2O72��(��ɫ)+H2O![]() 2CrO42��(��ɫ)+2H+����K2Cr2O7��Һ��������ʵ�飺
2CrO42��(��ɫ)+2H+����K2Cr2O7��Һ��������ʵ�飺
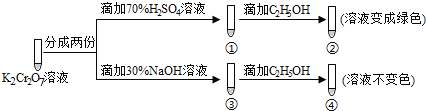
���ʵ�飬����˵��������ǣ� ��
A.������Һ��ɫ���������Һ���B.�Ա���������֪K2Cr2O7������Һ������ǿ
C.����C2H5OHʹCr2O72��������D.�������м���70%H2SO4��Һ����������Һ�ɱ�Ϊ��ɫ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com