
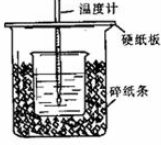
���� ��1���������ȼƵĹ������жϸ�װ�õ�ȱ�������������к��ȵIJⶨԭ��������
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�����
��3������Ӳֽ�壬����һ��������ɢʧ��
��4����Ӧ�ų����������������Լ�������Ķ����йأ��������к��ȵĸ����ʵ�����ش�
��5������������ʵ������ȷ�����Ҫ��֤һ��������ȫ��Ӧ������һ�����ʹ�����
��6��ȷ�����к��ȵ�ʵ����̣�һ��Ҫ�������¶ȣ��ȸ���Q=m•c•��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-$\frac{Q}{n}$kJ/mol�������Ӧ�ȣ�
��� �⣺��1�������ȼƵĹ����֪��װ�õ�ȱ�������ǻ��β�����������к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹����������ձ�Ϊһ���ߣ���������ɢʧ��
�ʴ�Ϊ�����β����������С�ձ��ںʹ��ձ���û��ƽ�룻
��2���к��Ȳⶨʵ��ɰܵĹؼ��DZ��¹�������С�ձ�֮��������ֽ���������Ǽ���ʵ������е�������ʧ��
�ʴ�Ϊ������ʵ������е�������ʧ��
��3�����ձ����粻��Ӳֽ�壬����һ��������ɢʧ����õ��к�����ֵ�����С��
�ʴ�Ϊ��ƫС��
��4����Ӧ�ų����������������Լ�������Ķ����йأ�����60mL 0.50mol/L�����50mL 0.55mol/L NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�������࣬���ų�������ƫ�ߣ������к��ȵľ���ǿ���ǿ�Ӧ����1molˮʱ�ų����ȣ��к�����ȣ�
�ʴ�Ϊ������ȣ���ȣ�
��5����ˮΪ����������Ϊ���ȹ��̣������ð�ˮ����ϡ����������Һ��Ӧ����Ӧ�ų�������ƫС����50mL 0.50mol/L NaOH��50mL 0.50mol/L���ᷴӦ������߲�����ȫ��Ӧ���ų�������������ֵҪС��
�ʴ�Ϊ��ƫС��ƫС��
��6���¶ȼ�Ҫ�ⷴӦǰ����Һ���¶ȣ��ⷴӦǰ����Һ���¶ȣ���Ϸ�Ӧ�������¶�һ��3�Σ���������������ʵ�飬��������ⶨ�¶�9�Σ�50mL 0.50mol/L������50mL 0.55mol/L NaOH��Һ�кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol=0.025mol����Һ������Ϊ��100ml��1g/ml=100g���¶ȱ仯��ֵ��TΪ��t2-t1����������0.025molˮ�ų�������ΪQ=m•c•��T=100g��4.18J/��g•�棩����t2-t1��=418��t2-t1��J����0.418��t2-t1��kJ������ʵ���õ��к��ȡ�H=-$\frac{0.418��t2-t1��kJ}{0.025mol}$��
�ʴ�Ϊ��9��-$\frac{0.418��t2-t1��kJ}{0.025mol}$��
���� ���⿼��ѧ���к��Ȳⶨ�����¹�����ʵ��ɹ��Ĺؼ�������ʵ��ԭ�����ɽ���ѶȲ���


 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������д���Ӧ�ã�
��̼���ƣ�Na2CO4����ϴ�ӡ�ӡȾ����֯����ֽ��ҽҩ�����������д���Ӧ�ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼�����Ƶĵ��뷽��ʽ��NaHCO3�TNa++H++CO${\;}_{3}^{2-}$ | |
| B�� | ��ͭ����������Ȼ�ͭ��Һ�����ӷ���ʽ��Cu2++2Cl-$\frac{\underline{\;���\;}}{\;}$Cu+Cl2�� | |
| C�� | ����ˮ������ӷ���ʽ��S2-+2H2O�TH2S+2OH- | |
| D�� | ��TiCl4�Ʊ�TiO2�Ļ�ѧ����ʽ��TiCl4+��x+2��H2O��������?TiO2•x H2O��+4HCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �ȼ����ؿ���������������Ĺ�����Դ���õ����CaΪ������������ˮLiCl-KCl�����������ʣ��ṹ����ͼ��ʾ��������ӦʽΪPbSO4+2Li++2e-=Li2SO4+Pb������˵������ȷ���ǣ�������
�ȼ����ؿ���������������Ĺ�����Դ���õ����CaΪ������������ˮLiCl-KCl�����������ʣ��ṹ����ͼ��ʾ��������ӦʽΪPbSO4+2Li++2e-=Li2SO4+Pb������˵������ȷ���ǣ�������| A�� | �ŵ�����У�Li+�������ƶ� | |
| B�� | �����µ�����Dz�����Ĺ��壬��ز����� | |
| C�� | ÿת��0.1 mol ���ӣ�����������20.7 g Pb | |
| D�� | �õ���ܷ�ӦΪ PbSO4+2LiCl+Ca=CaCl2+Li2SO4+Pb |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | OCS��CO��CO2��S�������ʵľ���������ͬ | |
| B�� | OCS�ڸ����·ֽ�ʱ��̼Ԫ�ػ��ϼ����� | |
| C�� | OCS�����к���2���Ҽ���2���м������Ǿ����ڷǼ��Լ� | |
| D�� | 22.4 L OCS��Լ����3��6.02��1023��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����ƿ�е�ԭ������ˮδ��ȥ | |
| B�� | �ܽ��õ��ձ�δ��ϴ�� | |
| C�� | ����ʱ�۲�Һ�温�� | |
| D�� | �ý�ͷ�ι�������ƿ��ˮʱ��ˮδ���̶���ֹͣ��ˮ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com