
���� ��1������m=nM������������������V=nVm������������Hԭ�����ʵ���Ϊ�����4�����ٸ���N=nNA������ԭ����Ŀ��
��2������n=$\frac{V}{{V}_{m}}$����HCl���ʵ������ٸ���c=$\frac{n}{V}$������Һ���ʵ���Ũ�ȣ�����ϡ�Ͷ��ɼ�����Ҫ1.5mol•L-1���Ȼ�����Һ�������
��3�����ݸ����ʵ����ʵ����Լ������к��е�ԭ�Ӹ��������
��� �⣺��1��0.5molCH4������=0.5mol��16g/mol=8g����״���µ����Ϊ��22.4L/mol��0.5mol=11.2L��0.5mol��������к��е���ԭ�ӵĸ���Ϊ��0.5mol��4��6.02��1023/mol=1.204��1024��
�ʴ�Ϊ��8��11.2��1.204��1024��
��2����״���µ�HCl����4.48L�������ʵ����ǣ�22.4L��22.4L/mol=1mol��������ЩHCl��������ˮ���Ƴ�200ml��Һ�������ʵ���Ũ���ǣ�c=1mol��0.2L=5mol/L��
����300 mL 0.5 mol•L-1�Ȼ�����Һ����Ҫ1.5 mol•L-1���Ȼ�����Һ�����Ϊ300 mL��0.5 mol•L-1��1.5 mol•L-1=100 mL��
�ʴ�Ϊ��5mol/L��100��
��3����0.5mol CO2���е����ʵ���Ϊ0.5mol��3=1.5mol��
�ڱ�״����22.4L �������ʵ���Ϊ$\frac{22.4L}{22.4L/mol}$=1mol��������ԭ�ӵ����ʵ���Ϊ1mol��1=1mol��
��4��18mLˮ������Ϊ18g�����ʵ���Ϊ$\frac{18g}{18g/mol}$=1mol��������ԭ�ӵ����ʵ���Ϊ1mol��3=3mol��
��0.2mol H2SO4���е����ʵ���Ϊ��0.2mol��6=1.2mol��
��������ԭ�ӵ����ʵ���Խ�����е�ԭ����Խ�࣬���е�ԭ�������ɴ�С˳�����е��Ǣۢ٢ܢڣ�
�ʴ�Ϊ���ۢ٢ܢڣ�
���� ���⿼�������ʵ��������ʵ���Ũ�ȵļ��㣬��Ŀ�Ѷ��еȣ���ȷ���ʵ��������ʵ���Ũ�ȡ�Ħ��������֮��Ĺ�ϵΪ���ؼ�������������ѧ���ķ�����������ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
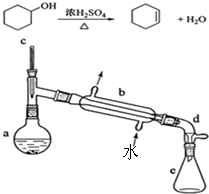 ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�ã��гּ����Ȳ�����ʡ�ԣ���ͼ��
ʵ���Һϳɻ���ϩ�ķ�Ӧ��ʵ��װ�ã��гּ����Ȳ�����ʡ�ԣ���ͼ��| ��Է������� | �ܶ� | �е� | �ܽ��� | |
| ���Ҵ� | 100 | 0.9618 | 161 | ����ˮ |
| ����ϩ | 82 | 0.8102 | 83 | ������ˮ |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
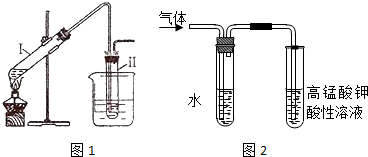
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������
���и�����������������ͼ��ʾת����ϵ���ǣ�ͼ�м�ͷ��ʾһ��ת������������| a | b | c | d | |
| �� | Si | SiO2 | H2SiO3 | Na2SiO3 |
| �� | N2 | NO | NO2 | HNO3 |
| �� | Cu | CuO | Cu��OH��2 | CuSO4 |
| �� | Na | NaCl | Na2CO3 | NaHCO3 |
| A�� | �٢� | B�� | �ڢ� | C�� | �ۢ� | D�� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ʵ�� ��� | HA���ʵ��� Ũ��/��mol•L-1�� | NaOH���ʵ��� Ũ��/��mol•L-1�� | ��Ϻ��� Һ��pH |
| �� | 0.1 | 0.1 | pH=a |
| �� | 0.12 | 0.1 | pH=7 |
| �� | 0.2 | 0.1 | pH��7 |
| �� | 0.1 | 0.1 | pH=10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
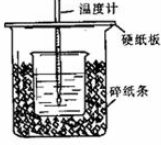
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش����⣺
�����������ض�����Ҫ�Ĺ�ҵ��Ʒ����ش����⣺�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com