
����Ŀ����֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ�����A��B��A��D�����ڱ���λ�����ڣ�Aԭ�Ӻ���������δ�ɶԵ��ӣ�BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض���Cԭ����ͬ����ԭ���а뾶���(ϡ���������)��E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ������������������Ϣ���ش��������⣺(����ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ)
��1��A��B��C��D����Ԫ�ص縺���ɴ�С����˳��Ϊ________________________________________��
��2��B���⻯��Ľṹ��____________________________����ռ乹��Ϊ____________________________________________________��
��3��E��������Ų�ʽ��__________________��E��ij�ֻ�����Ľṹ����ͼ��ʾ��
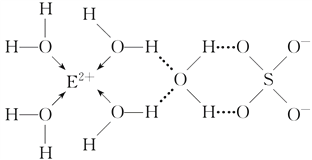
���������ð�����ѧ���ͷ��Ӽ�����������˻������и������Ӽ�����������______________________��
��4��A��B����̬�⻯��ķе�________���ߣ�A��D����̬�⻯��ķе�________���ߡ�
��5��A���ȶ��������У�����ԭ�ӵ��ӻ�����Ϊ________���ռ乹��Ϊ________��
���𰸡� N>C>Si>Na ![]() ������ 1s22s22p63s23p63d104s1��[Ar]3d104s1 ���Ӽ������ۼ�����λ������� NH3 SiH4 sp�ӻ� ֱ����
������ 1s22s22p63s23p63d104s1��[Ar]3d104s1 ���Ӽ������ۼ�����λ������� NH3 SiH4 sp�ӻ� ֱ����
��������������Ҫ������ӽṹ��A��B��D�����λ��Ϊ![]() ������ΪB�ĵ�һ�����ܱ�ͬ�������ڵ�����Ԫ�ش�����BΪ������ṹ��BΪN��AΪC��DΪSi��CΪNa��EΪ�������ڵ�Ԫ�أ������Ϊ1�����ӣ���������Ӳ����������E�ĵ����Ų�ʽΪls22s22p63s23p63d104s1��EΪCu��
������ΪB�ĵ�һ�����ܱ�ͬ�������ڵ�����Ԫ�ش�����BΪ������ṹ��BΪN��AΪC��DΪSi��CΪNa��EΪ�������ڵ�Ԫ�أ������Ϊ1�����ӣ���������Ӳ����������E�ĵ����Ų�ʽΪls22s22p63s23p63d104s1��EΪCu��
(1)A��B��C��D�ֱ�ΪC��N��Na��Si�����ݵ縺�Եĵݱ���ɿ�֪���縺��N>C>Si>Na��(2)B����̬�⻯��ΪNH3������ԭ��N��sp3�ӻ����ռ乹��Ϊ�����Ρ�
(3)EΪCu��������Ų�ʽΪ1s22s22p63s23p63d104s1������ͼʾ���ж�H2O���Ӻ�Cu2���������λ����ͬʱˮ����֮�仹���������H2O�����ڴ��ڹ��ۼ����û����ﻹ���������ӣ��������Ӽ���
(4)A��B����̬�⻯��ֱ�ΪCH4��NH3����е�ߵ�ΪNH3>CH4(��NH3����֮��������)��A��D����̬�⻯��ֱ�ΪCH4��SiH4����������ɺͽṹ���ƣ�SiH4����Է�����������CH4���ʷе�SiH4>CH4��
(5)CO2��Cԭ��sp�ӻ���CO2���ӳ�ֱ���Ρ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��Al2O3��Fe3O4��Al��Cu�е�ij���ַ�ĩ��϶��ɣ���Ƴɷַ����������£����з�������ȷ���ǣ� ��
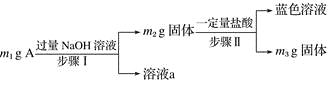
A. ��m1>m2ʱ����Һa��������ֻ��1��
B. ������ɫ��Һ�����ӷ���ʽ��Cu��2Fe3��===Cu2����2Fe2��
C. Ҫȷ����������Ƿ�Al����ȡA��������ϡHCl
D. ��m2��m3��2.96 g��Fe3O4����������Ϊ2.32 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��W��X��Y��Z��ԭ�������������ӵĶ���������Ԫ�ء�W����4ԭ�Ӻ�18���ӵ��⻯����ӣ�X�������������ǵ��Ӳ�����![]() ��Z��Wͬ���壬X��Y��Z������������֮��Ϊ9������˵����ȷ����
��Z��Wͬ���壬X��Y��Z������������֮��Ϊ9������˵����ȷ����
A. �����Ӱ뾶��W>X>Y>Z
B. ���⻯��ķе㣺Z>W
C. X2W��YZ��Ϊ���ӻ�����
D. W��X��Z����Ԫ����ɵĻ�����ˮ��Һ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ���ܹ۲쵽�����ЧӦ��һ�������ѧ���ͬѧ��ʵ�����Ʊ�Fe��OH��3���塣
��1��������������ɢϵ�ı��������ǣ�__��
��2���Ʊ�ʱ����_��εμ���_�У���Һ���_��ֹͣ���ȣ���Ӧ����ʽΪ__��
��3����һ���ٺ�ɫ�����ࣨSb2S3�����壬װ��U�ܣ�����缫��ֱͨ���磬�������������ٺ�ɫ������__����֤��Sb2S3������__��ɡ�
��4��������ʵ����֤�Ƶõ����������ǽ��壺__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)ʵ�����ý���ͭ��ϡ������ȡNO�����ӷ���ʽΪ______________________��
(2)NO���ж����壬ijѧ��Ϊ��ֹ��Ⱦ���÷�Һ©�����ձ�װ����һ���ġ����濪���á������ͣ��NO���巢��װ�ã���ͼ����ʾ��
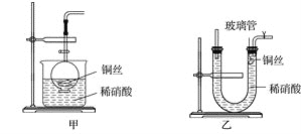
��ʵ������û��ͭ˿����ֻ��Сͭ������ʹ������װ�ý���ʵ��ʱ������˿״���ϰ���ͭ���Դ���ͭ˿����ʵ�飬����˿״���ϵijɷֿ�����________(�����)��
A���� B���� C���� D������
�ڴ�Һ©���Ļ���ʹ��Ӧ���У��ڷ�Һ©����ʵ�ʿ����������Ǻ���ɫ�ģ�ԭ����__________(�ѧ����ʽ)��
(3)Ϊ֤��ͭ˿��ϡ���ᷴӦ���ɵ�ȷʵ��NO��ijѧ���������һ����ͼ����ʾ��װ����ȡNO����Ӧ��ʼ������U�ι��Ҷ˹۲쵽��ɫ��NO���塣
�ٳ������ܵ�������______________________________________________________��
���÷�Ӧֹͣ�IJ���������ԭ����__________________________________________��
(4)�����ռ�NO�����װ�ã���������________(�����)��

(5)��32.64 gͭ��140 mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2 L����ش�
��NO�����Ϊ________ L��NO2�����Ϊ________ L��
�ڴ�����������ȫ���ͷź�����Һ�м���VmLamol��L��1��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ________ mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������̬����BCl3��CCl4��H2O��BeCl2�У�������ɴ�С��˳��Ϊ________��
��2������(H2S)���ӵĿռ乹��Ϊ________��������̼(CO2)���ӵĿռ乹��Ϊ________������(CH4)���ӵĿռ乹��Ϊ________��
��3�����ڰ�ˮ�ʹ���������Һ�������Խ����з�Ӧ�������Ȱ�(H2NCl)�����ӽṹ������NH3��H2NCl���ӵĿռ乹��Ϊ________������ʽΪ________��H2NCl���ȵĻ��ϼ�Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ������50Kg�Ľ����˴�Լ����2g����2g���������в����Ե��ʽ�����ʽ���ڣ�������Fe2+��Fe3+����ʽ�����������������ױ����գ���ƶѪ�߲�����ʱ��Ӧ���躬���������ӵĶ������Σ�����������(FeSO4)������ά����C����ʹʳ���е����������ӻ�ԭ�ɶ��������ӣ�����������������
(1) ������ά����C����ʹʳ�������������ӻ�ԭ�ɶ���������������仰ָ����ά����C����һ��Ӧ����_________��������________����
(2) ���ˮ�еμӱ��͵�FeCl3��Һ�Ʊ�Fe(OH)3���壬�������ӷ���ʽΪ��______________________��
(3) Ϊ����֤Fe3+�����ʣ�ij��ѧ��ȤС�����������ͼ��ʾ��һ��ʵ�飬ʵ�鷽����ƴ������_____(����ĸ)
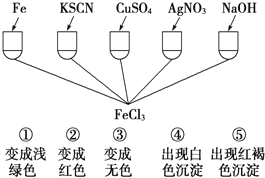
A. �ܺ͢� B. ֻ�Т� C. �ۺ͢� D. �٢ڢ�
(4) ��֪��������Cl2>Br2>Fe3+����ԭ��Fe2+>Br->Cl-������1L 0.2mol��L-1��FeBr2��Һ��ͨ���״��������2.24L����������������________���˷�Ӧ�����ӷ���ʽ��____________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�����ܱ�������,������Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
CO(g)+H2O(g) ��H����ƽ�ⳣ��(K)���¶�(T)�Ĺ�ϵ���±�:
T�� | 700 | 800 | 850 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�Իش���������:
��1��������ӦΪ____(��������������������)��Ӧ,�����¶�,ƽ����______ (��������Ӧ�������淴Ӧ��) �����ƶ���
��2��ij�¶���,�����Ϊ2L�ĺ����ܱ�������ͨ��2molCO2(g)��4molH2(B)��������Ӧ,5minʱ��Ӧ�ﵽƽ��,���CO2(g)��ת������75%��
��v(H2O)=______mol��L-1��min-l��
�ڸ��¶��·�Ӧ��ƽ�ⳣ��K=______.
��3������ˮú���Ĺ�������:
��C(s)+CO2(g) ![]() 2CO(g)��H1
2CO(g)��H1
��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H2
CO2(g)��H2(g) ��H2
�۷�Ӧ:CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��
CO(g)+H2O(g) ��H=________ (�ú���H1����H2�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������AlN����һ�����������ϣ��㷺Ӧ���ڵ��ӡ��մɵȹ�ҵ������һ�������£�AlN��ͨ����ӦAl2O3+N2+3C![]() 2AlN+3CO�ϳɣ���֪AlN����NaOH��Һ��Ӧ�ų��д̼�����ζ�����壮����������ȷ����
2AlN+3CO�ϳɣ���֪AlN����NaOH��Һ��Ӧ�ų��д̼�����ζ�����壮����������ȷ����
A. ������Ӧ�У�N2�ǻ�ԭ����Al2O3��������
B. ������Ӧ�У�ÿ����1molAlN��ת��6mol����
C. AlN�е�Ԫ�صĻ��ϼ�Ϊ+3
D. AlN��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��AlN+NaOH+H2O�TNaAlO2+NH3��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com