
| 1000�Ѧ� |
| M |
| n |
| V |
| 1.6g |
| 160g/mol |
| 4.66g |
| 233g/mol |
| 0.02mol |
| 0.1L |
| 0.04mol |
| 0.1L |
| 1000��1.20��36.5% |
| 36.5 |
| 6mol/L��0.1L |
| 12mol/L |


 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ﶼ�Dz�ͬԪ����ɵģ���ͬԪ����ɵ�����һ���ǻ����� |
| B���еĻ�ѧ��Ӧ�����ڻ��ϡ��ֽ⡢�û������ֽⷴӦ�е��κ�һ�� |
| C��һ��Ԫ�ؿ����ж����������ͬ�ּ�ֻ̬�ܶ�Ӧһ�������� |
| D��������ҺŨ��һ���Ȳ�������Һ��Ũ�ȴ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

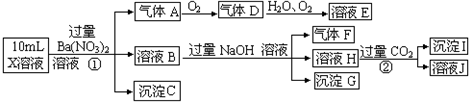
| A����ΪAlCl3����ΪNaHCO3 |
| B����Һ���л����Դ�������������У�Fe2+��NO3-��SO42- |
| C���ڼ��еμӶ���ʼ��Ӧ�����ӷ���ʽ��HCO3-+Ba2++OH-=BaCO3��+H2O |
| D����ɫ����A�����ܽ�����ҺD�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
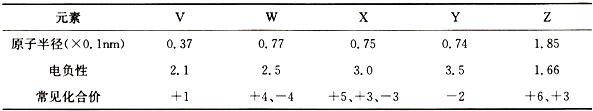
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com