
| ������ | K+ Na+ Fe2+ Ba2+ NH4+ |
| ������ | OH- NO3- I- HCO3- AlO2- HSO4- |
| ||
| ||


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���ܴ������е������� | H+��Ba2+��Al3+��NH4+��Fe2+ |
| ���ܴ������е������� | Cl-��SO42-��NO3-��CO32-��AlO2- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��KHSO4��Na2CO3����NH4��2SO4��FeCl3 |
| B��K2CO3��MgCl2��Al2��SO4��3��KOH |
| C��NaCl��KCl��CuCl2��AgNO3 |
| D��NaOH����NH4��2SO4��Na2SO4��BaCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
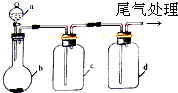 ʵ������ijЩ�������ȡ�����Ӽ��ռ�װ����ͼ��ʾ�����ô�װ�úͱ����ṩ������������ʵ�飬��������ǣ�������
ʵ������ijЩ�������ȡ�����Ӽ��ռ�װ����ͼ��ʾ�����ô�װ�úͱ����ṩ������������ʵ�飬��������ǣ�������
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
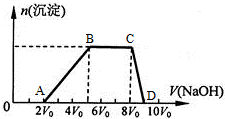
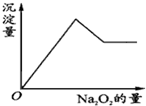 ��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ��
��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ij��Һ�еμ�BaCl2��Һ�ð�ɫ������ȡ�ð�ɫ������ϡ����ܽ�--ԭδ֪��Һ��һ������SO42- |
| B��ijδ֪�����ڿ�����ȼ�գ��������ʹ��ˮCuSO4����ɫ-ԭ����һ����H2 |
| C����ijδ֪��Һ�еμ����������ɫ���壬��������ͨ����������ʯ��ˮ�еð�ɫ����--ԭδ֪��Һ��һ������CO32- |
| D����ijδ֪��Һ�м���Ũ��NaOH��Һ�����ȣ�������ʹʪ��ĺ�ɫʯ����ֽ����������--ԭδ֪��Һ��һ������NH4+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ���ǣ�������
ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ���ǣ�������| ѡ�� | a������ | b������ | c���ռ����� | d������ |
| A | Ũ��ˮ | CaO | NH3 | H2O |
| B | Ũ���� | Ũ���� | HCl | NaOH��Һ |
| C | ϡ���� | Cu | NO2 | H2O |
| D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com