
27. ____ in a friendly way, they both were satisfied with the result.
A. Their quarrel settled
B. Their quarrel being settled
C. Having been settled their quarrel
D. After their quarrel settled
24. Have you told my father about the awarding ceremony?
– Yes, but he is too busy. I don’t think he ___. But in case he ____, let him sit between us.
A. is coming; is B. will come; does
C. would come; will D. comes; do
25 – Mary, where does the great noise come from?
-- A group of girls ___ football at the back of our house.
A.Play B.are playing
C.have played D.having been playing
26 According to a survey, many people can be successful ____ mainly because they believe too much in TV ads.
A.taken up B.taken over C.taken in D.taken off
22.My uncle promised to buy me a nice gift for my birthday, _____ beyond my imagination.
A. which B. that C. the one D. something
23 – Do you think John is coming to attend Mr.Lee ‘s lecture?
-- Sure, I have ___ him to .
A promised B suggested C demanded D persuaded
第一节 单项填空(15分)
21.Some of the exercises appear to be _____ones that you have done, but after taking____second look, you will find that they are different.
A. /, the B. the, the C. the, a D. /, a
25. 某校化学小组学生利用下图所列装置进行“铁与水反应”的实验,并利用产物进一步制取FeCl3·6H2O晶体。(图中夹持及尾气处理装置均已略去)
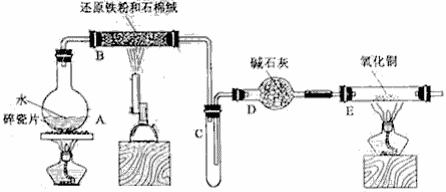
(1)装置B中发生反应的化学方程式是 。
(2)装置E中的现象是 。
(3)停止反应,待B管冷却后,取其中的固体,加入过量稀盐酸充分反应,过滤。简述检验滤液中Fe3+的操作方法:
(4)该小组学生利用上述滤液制取FeCl3·6H2O晶体,设计流程如下:
滤液→FeCl3溶液→FeCl3·6H2O晶体
①步骤I中通入Cl2的作用是 。
②步骤II从FeCl3稀溶液中得到FeCl3·6H2O晶体的主要操作包括: 。
③该流程中需保持盐酸过量,主要原因是(结合离子方程式简要说明) 。
24.明矾水溶液呈____性,原因是_ __ ;小苏打水溶液呈____性,原因是_ _ _.
把上述两溶液混合后呈现的现象有 _,反应现象的离子方程式是_ __ .
23. 水玻璃在工业上可作粘合剂,当它与NH4Cl溶液接触时,会很快凝结。原因是
22. 有一不饱和的NaOH溶液。加入a mol Na2O、b mol Na2O2或c mol NaOH恰好得到NaOH饱和溶液(同一温度下).则a、b、c的关系为
A.a=b=c/2 B.a=b>c/2 C.a=b<c/2 D.a>b>c
21. Al3+ + 3H2O
Al(OH)3 +3 H+ 的平衡体系中,要使平衡向水解方向移动,且使溶液的pH值增大,应采取的措施是
Al3+ + 3H2O
Al(OH)3 +3 H+ 的平衡体系中,要使平衡向水解方向移动,且使溶液的pH值增大,应采取的措施是
A.加热 B.加适量NaOH溶液 C.通入氯化氢气体 D.加入固体三氯化铝
20. 0.02mol·L-1的HCN溶液与0.02mol·L-1的NaCN溶液等体积混合,已知混合液中[CN-]<[Na+],则下列关系正确的是
A.[Na+]>[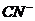 ]>[
]>[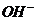 ]>[H+] B.[HCN]+[
]>[H+] B.[HCN]+[ 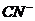 ]=0.04
mol·L-1
C.[Na+]+[H+]=[
]=0.04
mol·L-1
C.[Na+]+[H+]=[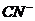 ]+[
]+[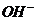 ] D.[
] D.[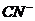 ]>[HCN]
]>[HCN]
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com