
| ʵ����� | ʵ������ | ʵ���� |
| 1 | ��AgNO3 ��Һ | �а�ɫ�������� |
| 2 | ������NaOH ��Һ������ | �ռ�������1.12L��������ɱ�״���µ������ |
| 3 | ������BaCl2 ��Һ�������ó�������ϴ�ӡ������������������м�����ϡ���ᣬȻ�������� | ��һ�γ�������Ϊ6.27g���ڶ��γ�������Ϊ2.33g |
| �����ӷ��� | ���ʵ���Ũ�ȣ�mol?L-1�� |
| 2.33g |
| 233g/mol |
| 0.01mol |
| 0.1L |
| 3.94g |
| 197g/mol |
| 0.02mol |
| 0.1L |
| 1.12L |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
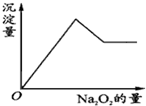 ��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ��
��һ����Һ�����ܺ���Al3+��Fe3+��K+��NH4+��Mg2+��Cu2+�������е�һ�ֻ��֣��ּ���Na2O2��ĩֻ����ɫ��ζ������ų�����ͬʱ������ɫ����������Na2O2���������ɰ�ɫ��������֮��Ĺ�ϵ��ͼ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
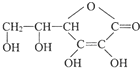
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ˮ�м������NaOH��[Na+]=[Cl-]+[ClO-]+[OH-] |
| B��pH=8.3��NaHCO3��Һ��[Na+]��[HCO3-]��[CO32-]��[H2CO3] |
| C��pH=11�İ�ˮ��pH=3������������ϣ�[Cl-]=[NH4+]��[OH-]=[H+] |
| D��0.2mol?L-1CH3COOH��Һ��0.1mol?L-1NaOH��Һ�������ϣ�2[H+]-2[OH-]=[CH3COO-]-[CH3COOH] |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������10 s |
| B������12 s |
| C������12 s |
| D����12 s |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
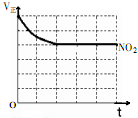 ���Ǵ����к����ḻ��һ��Ԫ�أ���ش����к����仯�����������⣺
���Ǵ����к����ḻ��һ��Ԫ�أ���ش����к����仯�����������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������� | B������ |
| C�������� | D����֬ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com