
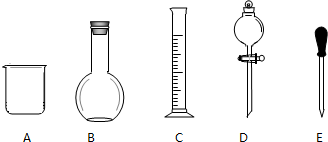
 ʵ������Ҫ0.1mol/LNaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮
ʵ������Ҫ0.1mol/LNaOH��Һ480mL��0.5mol/L��������Һ500mL��������������Һ����������ش��������⣮| n |
| V |
| 1000�Ѧ� |
| M |
| n |
| V |
| n |
| V |
| 1000��1.84g/ml��98% |
| 9g/mol |


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������ | H+��Na+��Al3+��Ag+��Ba2+ |
| ������ | OH-��Cl-��CO32-��NO3-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ������/kJ?mol-1 | I1 | I2 | I3 | I4 |
| A | 932 | 1821 | 15390 | 21771 |
| B | 738 | 1451 | 7733 | 10540 |
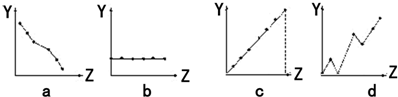
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
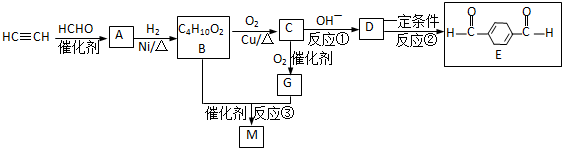
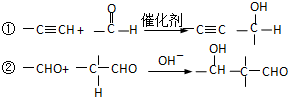
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��1mol/L |
| B��2.5mol/L |
| C��5mol/L |
| D��2mol/L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��0.1 mol?L-1 |
| B��1 mol?L-1 |
| C��3 mol?L-1 |
| D��1.5 mol?L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ʹ���ȳʻ�ɫ����Һ��Na+��NO-3��I-��K+ |
| B����������Al3+����Һ��Na+��NO-3��SO2-4��HCO-3 |
| C������ˮ�������c��H+��=10-12mol?L-1����Һ��K+��Ba2+��Cl-��Br- |
| D��������Al������������Һ��K+��SO2-4��Cl-��NO-3 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com