
第一节:单项填空(共15小题;每小题1分,共15分)
11. --- Do you know if Terry will go camping this weekend?
--- Terry? Never! She ______ tents and fresh air!
A. has hated B. hated C. will hate D. hates
12. I wonder what difficulty they have had _____ her to leave.
A. to persuade B. persuaded C. persuading D. persuade
13. _____ a lot of other countries, we’re in an economic recession(衰落).
A. Having a lot in common B. Having in common with
C. In common with D. In common
14. Now that we _____ all the money, it’s no use saying it’s all my fault.
A. had lost B. lost C. have lost D. lose
15. That big armchair will have to go: it _____ too much space in the small study.
A. takes up B. makes up C. saves up D. puts up
16. _____ good, the apples were soon sold out.
A. Tasting B. Taste C. Tasted D. Having tasted
17. The man lying on the ground _____ to me that his distinctive cock had _____ several eggs.
A. lay;lain B. lied; lied C. lied; laid D. lied;lain
18. As soon as she entered the room, the girl caught sight of the flowers _____ by her mother.
A. buying B. being bought C.. were bought D. bought
19. That movie is about a love story _____ a(n)_____ train to Tokyo.
A. setting in ; express B. set in; express C. sets in; fast D. setting in; fast
20. When _____ different cultures, we often pay attention only to the differences without noticing the many similarities.
A. compared B being compared C. comparing D. having compared
21. --- Have you sold your house?
--- No. Last week only four people came to look at the house, _____ wanted to buy it.
A. none of them B. none of whom C. neither of them D. neither of whom
22. Generally speaking, employers should not _____ themselves in the private affairs of their staff.
A. remind B. inform C. understand D. involve
23. --- Is it OK to go Dutch for this great dinner?
--- _____.
A. Don’t be nervous. I’ll arrange. B. No, you should go thee first.
C. Forget it. It’s my treat today. D.OK. It’s my turn to serve today.
24. Humans differ _____ other mammals _____ their ability to speak.
A. with; in B. from; about C. with; on D. from; in
25. From the _____ look of the boys, I found they were _____.
A. satisfying; satisfied B. satisfying; satisfying
C. satidfied; satisfied D. satisfied; satisfying
第二节
请听下面两段对话,选出最佳选项。请听第六段材料,回答6、7题。
6. When will the plane take off?
A. At 5:00. B. At 4:30 C. At 5:30
7. What’s the woman probably going to do in Washington?
A. Study there B. Work there. C. Visit her sister.
请听第七段材料,回答第8至第10题
8. How many cigarettes does the man smoke every day?
A. About 15 B. About 20 C. About 30
9. What will the man do tomorrow?
A. Begin to quit smoking. B. See a doctor. C. Take an exam.
10. What can we know about the man from the conversation?
A. He has a bad headache. B. He has quitted smoking.
C. He usually works late at night.
英语知识运用
第一节(共10个小题,每小题1分,共10分)
请听下面五段对话,选出最佳选项。
1. What does the man want from the woman?
A. A paintbrush. B. A picture. C. A hammer.
2. Where does the woman come from?
A. San Francisco. B. Paris. C. Rome.
3. When will the school term begin?
A. On September12. B. On September 20. C. On September 22.
4. What can we know from the conversation?
A.Tom’s parents have left Los Angeles.
B. The woman called on Tom’s parents last night.
C. Tom’s parents have gone to Los Angeles.
5. How long did it take the man to go to work on foot?
A. About fifteen minutes. B. About an hour. C. About forty-five minutes.
23.(10分)化学实验是探究化学原理的主要手段。
(1)为了探究钠跟非金属的反应,有人进行如下实验:观察用刀切开的钠的表面所发生的变化。把一小块钠放在石棉网上加热,观察发生的现象。
从钠的化学性质角度分析你认为通过本实验可探究得出什么结论?
。
实验中主要用了什么方法? 。
(2)为了探究钠跟水的反应,进一步进行两个实验:
①向一个盛有水的小烧杯里滴入几滴酚酞试液,然后把绿豆大的一块钠投入小烧杯中。
②切一小块绿豆大的钠,用铝箔(事先用针刺一此小孔)包好,再用镊子夹住,放在试管口下,用排水法收集气体。待试管中气体集满时,小心取出试管,把试管口向下移近酒精灯火焰,检验生成的气体。
根据实验①的现象可判断,钠跟水反应后的产物之一是 ,根据实验②的现象判断,钠跟水反应的产物之一是 ,综合起来可得钠跟水反应的化学反应原理,请用离子方程式表示 。24.(10分)A、B、C、D是按原子序数由小到大排列的第二、三周期元素的单质。B、E均为组成空气的成分。F的焰色反应呈黄色。在G中,非金属元素与金属元素的原子个数比为1:2。在一定条件下各物质之间的相互转化关系如下图(图中部分产物未列出):
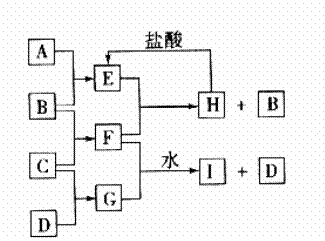
请填写下列空白
(1)A是 ,C是 。(写化学式)
(2)H与盐酸反应生成E的化学方程式是 。
(3)E与F反应的化学方程式是 。
(4)F与G的水溶液反应生成I和D的离子方程式是
;
22.(8分)用电弧法合成的储氢纳米碳管常伴有大量的碳纳米颗粒(杂质),这种颗粒可用氧化气法提纯,其反应式为:
(1)配平下列反应的化学方程式:







 C+ K2Cr2O7+ H2SO4 CO2↑+ Cr2(SO4)3+ K2SO4+ H2O
C+ K2Cr2O7+ H2SO4 CO2↑+ Cr2(SO4)3+ K2SO4+ H2O
(2)反应的氧化剂是 ,氧化产物是 ;
(3)H2SO4在上述反应中表现出来的性质是 (填选项编号)
A.酸性 B.氧化性 C.吸水性 D.脱水性
(4)上述反应中若产生0.2molCO2气体,则转移电子的物质的量是 mol。
21.(12分)现有FeCl3溶液,根据以下现象将所发生反应的离子方程式填入相应的横线上:
(1)加入甲基橙变红是因为 ;
。
(2)加入铁屑,溶液颜色变浅绿色 ;
。
(3)滴入沸水中变红褐色 ;
(4)加入KSCN溶液变血红色 ;
(5)加入氨水,产生红褐色沉淀 ;
(6)加入H2S气体,产生淡黄色浑浊
。
20.下列溶液中,溶质的物质的量浓度为1mol·L-1的是( )
A.将40gNaOH溶于1L水所得的溶液 B.将80gSO3溶于水并配成1L的溶液
C.将0.5mol·L-1的NaNO3溶液100mL加热蒸发掉50g水的溶液
D.含K+为2mol的K2SO4溶液
Ⅱ卷(40分)
19.向含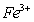 、
、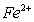 、
、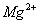 、
、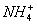 的水溶液中,加入足量的Na2O2固体,充分反应后再加入过量的稀盐酸,上述离子数目没有变化的是( )
的水溶液中,加入足量的Na2O2固体,充分反应后再加入过量的稀盐酸,上述离子数目没有变化的是( )
A. 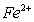 B.
B. 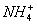 C.
C. 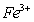 D.
D. 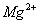
18.如图所示,A处通入Cl2,关闭B阀时,C处的湿润红色布条看不到明显现象,打开B阀后,C处红色布条逐渐褪色,则D瓶中装的是( )
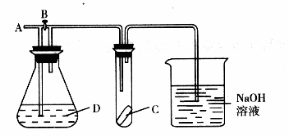
A.浓H2SO4 B.NaOH溶液 C.浓盐酸 D.饱和NaCl溶液
湖北省互联网违法和不良信息举报平台 | 网上有害信息举报专区 | 电信诈骗举报专区 | 涉历史虚无主义有害信息举报专区 | 涉企侵权举报专区
违法和不良信息举报电话:027-86699610 举报邮箱:58377363@163.com